
Preparing for an upcoming test in the field of natural sciences requires a solid understanding of key principles and methodologies. Mastering the fundamental ideas and practicing relevant problems are crucial steps to success. Whether you’re reviewing theoretical concepts or applying them in practical scenarios, a comprehensive approach ensures you grasp all the necessary material.
Focus on the core principles and essential topics that frequently appear in assessments. Pay attention to the specific terminology and techniques that can make or break your performance. With proper focus and consistent practice, any complex topic can be broken down into manageable sections.
Throughout your preparation, strategic planning will help you approach each section with clarity. It’s not just about memorizing facts; it’s about understanding how to apply them in different contexts. Whether you’re solving problems, interpreting data, or analyzing theoretical concepts, a structured study plan can guide you towards achieving a well-rounded grasp of the material.
Essential Insights for Exam Preparation
Effective preparation for an upcoming test in the natural sciences involves understanding core concepts, practicing key problem-solving techniques, and applying theoretical knowledge to practical scenarios. Mastering these elements will help you navigate various types of questions with confidence, ensuring that you are well-prepared to tackle any challenge that comes your way.
Key Topics to Focus On
To perform well, it is essential to focus on the most significant topics that are likely to appear in assessments. Concentrate on fundamental principles such as atomic structure, bonding, reactions, and periodic trends. Make sure you are comfortable with balancing equations, calculating mole ratios, and identifying various types of chemical reactions. Understanding these core subjects will give you a solid foundation for more complex material.
Effective Study Techniques
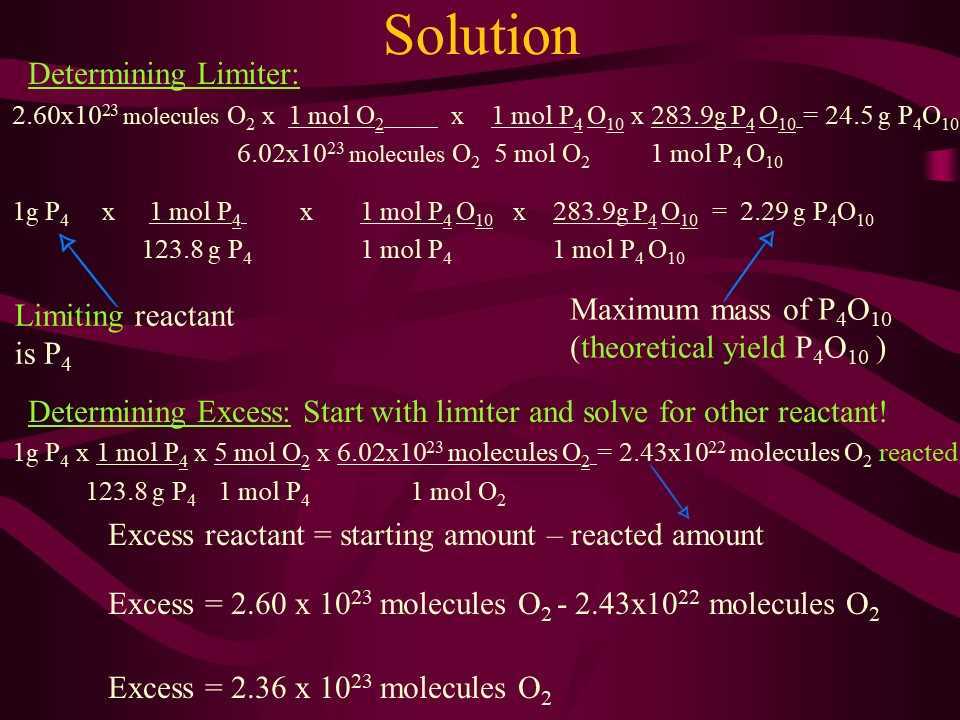
Rather than cramming information at the last minute, break down your study sessions into manageable chunks. Use active recall to test your understanding, and consider forming study groups to exchange insights. Practice solving problems under timed conditions to simulate the pressure of the test environment. This will help you improve your speed and accuracy while reinforcing your knowledge.
Key Concepts to Remember for Science Test
To succeed in any assessment related to the natural sciences, it is crucial to grasp the fundamental principles that form the backbone of the subject. These core ideas will help you not only understand complex topics but also apply them effectively when answering questions. A deep understanding of these concepts is essential for tackling both theoretical and practical challenges.
Atomic structure is one of the building blocks you should focus on. Familiarize yourself with protons, neutrons, electrons, and their roles in forming atoms and molecules. Understanding how elements interact on a subatomic level will allow you to comprehend more complex phenomena such as bonding and reactions.
Equally important is the periodic table, which provides a wealth of information about element properties and trends. Recognizing patterns in atomic size, electronegativity, and ionization energy can help you predict how elements will behave in chemical processes. These relationships are key to answering questions involving element classification and chemical reactivity.
Important Chemical Reactions to Study

Mastering key chemical transformations is essential for performing well in any assessment related to the natural sciences. Certain reactions appear frequently and form the foundation for understanding how different substances interact. By familiarizing yourself with these fundamental processes, you’ll be able to approach problems with confidence and accuracy.
Here are some critical reactions you should focus on:
- Combustion Reactions: These reactions involve the burning of a substance in the presence of oxygen, typically producing energy, carbon dioxide, and water. Understanding the stoichiometry of combustion is key.
- Synthesis Reactions: A process where two or more reactants combine to form a single product. These are important for understanding how compounds are created.
- Decomposition Reactions: In these reactions, a single compound breaks down into two or more simpler substances. This process is crucial in analyzing the stability of compounds.
- Single Replacement Reactions: One element displaces another in a compound. These reactions are important for understanding reactivity and ion exchange.
- Double Replacement Reactions: Two compounds exchange components to form new products, often leading to the formation of a precipitate, gas, or water.
- Acid-Base Reactions: These involve the transfer of protons between an acid and a base. Understanding the concepts of neutralization and pH balance is essential for solving many related problems.
Incorporating these reactions into your studies will provide a strong foundation for tackling various questions and applying your knowledge effectively. Be sure to practice balancing equations and predicting the products of different reactions to solidify your understanding.
Understanding Atomic Structure and Bonding

A deep understanding of atomic structure and how atoms interact to form compounds is essential for grasping many concepts in the natural sciences. The arrangement of particles within atoms and the way they bond to each other governs the properties of all substances. This section explores the fundamental principles of atomic theory and the types of bonds that hold molecules together.
Atomic Structure Basics
At the core of every atom is a nucleus composed of protons and neutrons, surrounded by electrons that occupy specific energy levels. The number of protons determines the element, while the electrons are responsible for chemical behavior. Understanding electron configuration and the concept of valence electrons is crucial for predicting how atoms will bond and interact.
Types of Chemical Bonds
Atoms combine through different types of bonds, each with distinct characteristics. The two most common types are ionic and covalent bonds, but metallic bonds also play a significant role in solid materials. The type of bond formed affects the physical properties of the compound, such as its melting point, solubility, and electrical conductivity.
| Bond Type | Electron Sharing | Example | Properties |
|---|---|---|---|
| Ionic | Electrons are transferred from one atom to another | NaCl (Sodium Chloride) | High melting point, conducts electricity in solution |
| Covalent | Electrons are shared between atoms | H2O (Water) | Low melting point, does not conduct electricity |
| Metallic | Electrons are free to move across atoms | Cu (Copper) | Good electrical conductor, malleable |
By understanding the structure of atoms and the nature of the bonds between them, you’ll be able to explain the behavior of substances in a variety of chemical reactions and scenarios.
Common Acids and Bases Explained
Acids and bases are fundamental components in many chemical reactions and play a crucial role in determining the behavior of substances in different environments. Understanding how these substances interact with one another, as well as their properties, is essential for grasping a wide range of chemical processes. This section covers the basics of common acids and bases, including their characteristics and applications.
Acids are substances that donate protons (H⁺ ions) in a reaction, while bases accept them. This simple interaction forms the basis of numerous chemical processes, from neutralization reactions to pH changes. Below are some common examples of acids and bases, as well as their typical uses:
Common Acids
- Hydrochloric Acid (HCl): Found in stomach acid, it plays a key role in digestion and is commonly used in cleaning and industrial applications.
- Acetic Acid (CH₃COOH): A key component of vinegar, acetic acid is used in food preservation and as a cleaning agent.
- Sulfuric Acid (H₂SO₄): This strong acid is widely used in manufacturing, particularly in the production of fertilizers, dyes, and detergents.
- Citric Acid (C₆H₈O₇): Found naturally in citrus fruits, it is often used in food processing, cosmetics, and pharmaceuticals.
Common Bases
- Sodium Hydroxide (NaOH): Also known as lye, this base is commonly used in soap making, drain cleaners, and as a strong industrial chemical.
- Ammonium Hydroxide (NH₄OH): A water solution of ammonia, it is used in household cleaning products and in agriculture as a fertilizer.
- Calcium Hydroxide (Ca(OH)₂): Often used in water treatment and as a neutralizing agent in industrial processes.
- Magnesium Hydroxide (Mg(OH)₂): Commonly known as milk of magnesia, it is used as an antacid and laxative.
Understanding the properties and uses of these acids and bases is vital for solving problems related to pH, neutralization, and various other chemical reactions.
Organic Fundamentals for Beginners
Understanding the basics of organic compounds is essential for anyone starting out in the study of natural sciences. Organic molecules, primarily composed of carbon, hydrogen, and oxygen, are the building blocks of life and are involved in countless chemical reactions. This section will introduce the fundamental concepts and types of compounds, providing a foundation for further exploration in this field.
Organic compounds are categorized based on their functional groups and structures. These groups of atoms determine the chemical properties and reactivity of the molecules. Below are some key points to help you grasp the essential elements of organic molecules:
Key Types of Organic Compounds
- Hydrocarbons: Compounds made up only of carbon and hydrogen atoms. These can be further divided into alkanes, alkenes, and alkynes based on the types of bonds between carbon atoms.
- Alcohols: Organic compounds containing one or more hydroxyl (-OH) groups. These are commonly found in beverages, cleaners, and pharmaceuticals.
- Carboxylic Acids: Compounds with a carboxyl group (-COOH). They are commonly used in the production of plastics, food preservation, and as industrial solvents.
- Amines: Nitrogen-containing compounds that play important roles in the synthesis of proteins and pharmaceuticals.
- Esters: Derived from an alcohol and a carboxylic acid, these compounds are responsible for the aromas in many fruits and flowers.
Basic Principles of Organic Reactions
- Addition Reactions: In these reactions, atoms are added to a molecule, typically breaking a double or triple bond in the process. A common example is the addition of hydrogen to an alkene.
- Substitution Reactions: A functional group in a molecule is replaced by another. This is commonly seen in halogenation reactions, where a hydrogen atom is replaced by a halogen.
- Elimination Reactions: These involve the removal of atoms or groups from a molecule, leading to the formation of a double or triple bond.
- Rearrangement Reactions: In these reactions, atoms or groups within a molecule shift positions, leading to a different structural configuration.
Mastering these fundamental concepts will allow you to better understand the vast array of organic molecules and their interactions in various chemical processes.
Balancing Chemical Equations Simplified
One of the core principles of studying chemical reactions is ensuring that the equation representing the process is balanced. This means that the number of atoms of each element must be the same on both sides of the equation. Balancing equations helps us follow the law of conservation of mass, which states that matter cannot be created or destroyed in a chemical reaction. By learning to balance equations, you gain a deeper understanding of how reactions occur and the quantities involved.
To balance an equation, start by identifying the reactants and products, then adjust the coefficients (the numbers in front of the chemical formulas) to ensure that each element is present in equal amounts on both sides. Below is a simplified approach to balancing equations:
Steps for Balancing Equations
- Write down the unbalanced equation.
- Count the number of atoms of each element on both sides of the equation.
- Start by balancing the elements that appear in only one reactant and one product.
- Adjust coefficients to balance atoms, starting with the most complex molecules.
- Finally, balance hydrogen and oxygen atoms, as these are often found in multiple compounds.
- Double-check the balance by counting the atoms of each element again.
Here’s an example of a simple balanced equation:
| Unbalanced Equation | Balanced Equation |
|---|---|
| H₂ + O₂ → H₂O | 2H₂ + O₂ → 2H₂O |
By practicing these steps, you will become more comfortable with balancing equations, which is crucial for solving problems in chemical reactions and understanding stoichiometry.
Periodic Table Trends and Their Importance
The periodic table is more than just a list of elements; it reveals patterns that help us understand the properties and behaviors of elements. These trends, which describe how certain characteristics change across periods and down groups, are crucial for predicting chemical reactions and understanding the nature of substances. By studying these patterns, scientists can make sense of the behavior of elements, predict reactions, and apply this knowledge in various scientific fields.
Among the most significant trends are atomic radius, ionization energy, electron affinity, and electronegativity. These properties provide valuable insights into how elements interact and bond with one another, shaping the way we approach chemical equations and molecular structures.
Key Trends in the Periodic Table
- Atomic Radius: This refers to the size of an atom. As you move across a period, the atomic radius decreases due to an increase in nuclear charge, which pulls electrons closer. However, as you move down a group, the atomic radius increases because of the addition of electron shells.
- Ionization Energy: This is the energy required to remove an electron from an atom. Ionization energy generally increases across a period due to the stronger attraction of the nucleus to the electrons, while it decreases down a group as the outer electrons are further from the nucleus.
- Electron Affinity: This is the energy change when an electron is added to an atom. Generally, electron affinity becomes more negative across a period as elements gain electrons more readily, but it becomes less negative down a group.
- Electronegativity: This property measures an atom’s ability to attract electrons in a chemical bond. Electronegativity increases across a period and decreases down a group, which explains why elements in the same group tend to behave similarly in reactions.
Understanding these trends is not only essential for academic purposes but also for practical applications in industries like medicine, materials science, and environmental studies. Recognizing the predictable nature of these trends allows chemists and engineers to design new materials, synthesize drugs, and create more efficient energy sources.
Common Lab Techniques and Procedures
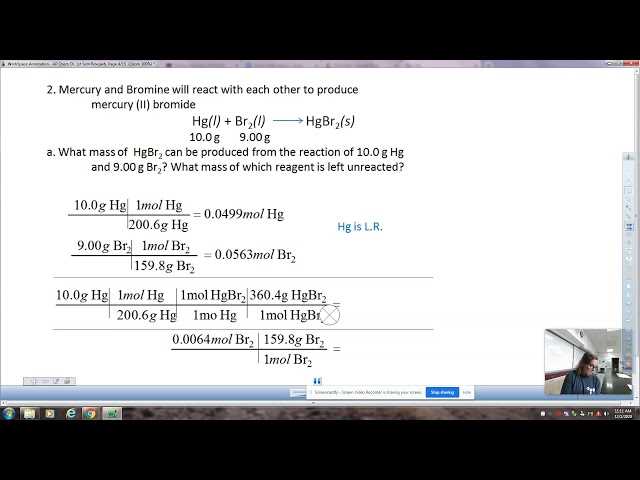
In the laboratory, there are several essential techniques that are routinely used to conduct experiments and gather accurate data. Mastery of these methods is crucial for obtaining reliable results, ensuring safety, and maintaining proper scientific standards. These procedures range from simple measurements to complex manipulations of substances, and they are the foundation for most experimental work.
Below are some of the most common techniques and procedures that are frequently used in scientific laboratories:
Key Laboratory Techniques
- Filtration: This technique is used to separate solid particles from liquids or gases. Filtration is commonly performed using a filter paper in a funnel or other filtering apparatus.
- Distillation: Distillation is used to separate liquids based on their boiling points. This process is especially useful for purifying liquids or separating mixtures of liquids.
- Titration: A method to determine the concentration of a substance in a solution by adding a reagent of known concentration until a reaction is complete. This is commonly used in acid-base reactions.
- Recrystallization: This is used to purify solid compounds. It involves dissolving the substance in a hot solvent and then slowly cooling the solution to allow pure crystals to form.
- Volumetric Analysis: This involves measuring the volume of a liquid reagent required to react with a sample, and it is typically used in quantitative analysis for determining concentrations of unknown substances.
- Separation Techniques: Methods such as centrifugation, chromatography, and evaporation are used to separate mixtures based on differences in physical properties like density, solubility, or boiling point.
Essential Safety and Handling Procedures
- Proper Handling of Chemicals: Always wear appropriate protective gear such as gloves, goggles, and lab coats when handling chemicals. Be familiar with the properties and hazards of substances before use.
- Use of Lab Equipment: Knowing how to properly use and maintain lab equipment is crucial for achieving accurate results. Always follow standard operating procedures for instruments like pipettes, burettes, and spectrophotometers.
- Waste Disposal: Proper disposal of chemical waste is critical to maintaining a safe lab environment. Always follow guidelines for the disposal of chemicals and other materials.
By mastering these fundamental techniques and adhering to laboratory safety protocols, you can ensure that experiments are conducted effectively and safely, leading to accurate and reproducible results.
Practice Problems for Effective Learning
One of the most effective ways to reinforce knowledge and develop problem-solving skills is through regular practice. By working through different problems, students can test their understanding of key concepts, identify areas of weakness, and gain confidence in applying theoretical knowledge to practical situations. Practice problems offer an excellent opportunity to improve critical thinking and reinforce the learning process.
Engaging in exercises not only helps solidify concepts but also prepares individuals for future challenges. Whether it’s solving quantitative problems, balancing equations, or interpreting experimental data, practice enhances both accuracy and speed in tackling various tasks.
Types of Practice Problems
- Conceptual Questions: These problems test understanding of principles and theories without requiring complex calculations. They are helpful for reinforcing the underlying concepts.
- Calculation-Based Problems: These require applying formulas and mathematical operations to solve real-world scenarios, improving problem-solving efficiency and accuracy.
- Application Problems: These simulate real-life situations, where knowledge is applied to solve practical problems, such as in laboratory settings or industrial processes.
- Data Interpretation: These focus on analyzing and interpreting experimental data, graphs, and results, helping to develop analytical thinking skills.
Example Practice Problems
| Problem Type | Example Problem | Solution Approach |
|---|---|---|
| Conceptual | Explain the difference between ionic and covalent bonding. | Compare the characteristics of electron transfer and sharing in bonds. |
| Calculation-Based | Calculate the molarity of a solution prepared by dissolving 5 grams of sodium chloride in 250 mL of water. | Use the formula M = moles/volume and convert grams to moles before calculating. |
| Application | Design an experiment to measure the rate of a chemical reaction under varying temperatures. | Outline the experimental steps and variables, then suggest data collection methods. |
| Data Interpretation | Interpret the results of a titration experiment to determine the concentration of an unknown acid. | Analyze the volume of titrant used and apply stoichiometric calculations to find the concentration. |
Regularly solving practice problems helps sharpen skills, prepares individuals for more complex challenges, and ensures that knowledge is well understood and retained for future application.
Essential Calculations in Chemistry
In scientific studies, particularly those involving substances and their interactions, performing accurate calculations is a vital skill. These calculations are used to quantify elements, measure concentrations, determine reaction yields, and predict various properties of materials. Mastery of these essential mathematical processes is crucial for drawing meaningful conclusions from experimental data and ensuring the validity of results.
Several key calculations form the foundation of many scientific procedures. From determining the amount of a substance in a mixture to calculating the heat released or absorbed in a reaction, these formulas provide the means to analyze and interpret data with precision.
Some of the fundamental calculations include molar mass determination, stoichiometric conversions, concentration calculations, and equilibrium constant evaluations. These processes are often used to solve real-world problems in fields like environmental science, medicine, and materials engineering.
Important Chemical Safety Guidelines
Working with substances in any scientific or industrial setting requires strict adherence to safety protocols to prevent accidents and ensure the well-being of everyone involved. Proper handling, storage, and disposal of materials, as well as using protective equipment, are essential steps in maintaining a safe working environment. Understanding and applying these safety guidelines can help avoid harmful exposures, chemical reactions, and environmental hazards.
Key safety measures include wearing appropriate personal protective equipment (PPE), such as gloves, goggles, and lab coats, to minimize direct contact with potentially dangerous substances. Ensuring proper ventilation, handling chemicals in designated areas, and being aware of the specific hazards of each substance are also critical practices. Additionally, knowing how to react in case of spills, accidents, or exposure is vital for preventing injury or contamination.
Familiarity with safety data sheets (SDS), which provide essential information about chemical properties and handling instructions, is crucial for anyone working with hazardous materials. Regular training and a proactive safety culture in the workspace contribute to minimizing risks and promoting safe practices for everyone involved.
Tips for Memorizing Chemical Formulas
Memorizing formulas can be a challenge, especially when dealing with numerous elements and compounds. However, with the right techniques, it becomes much easier to retain and recall these formulas when needed. Understanding the structure and composition of molecules can help simplify the process, as well as using mnemonic devices and visual aids to reinforce memory.
Break it Down: Start by breaking down complex formulas into smaller parts. For instance, consider the molecular formula for water, H2O. Instead of memorizing it as one entity, focus on the individual components – the two hydrogen atoms and one oxygen atom. This makes the information more digestible.
Use Mnemonics: Mnemonics are powerful tools for memory. Create a sentence or phrase using the first letters of the elements in a compound. For example, to remember the formula for methane (CH4), you might use the phrase “Carbon Holds Four” to remind you that carbon (C) bonds with four hydrogen (H) atoms.
Practice Regularly: Repetition is key when memorizing formulas. Regularly writing them down or using flashcards can reinforce your memory and help you recall them quickly. Testing yourself periodically can also help gauge your progress.
Visualize the Structure: Sometimes, drawing the molecular structure of a compound can help reinforce the formula. By visualizing how atoms are arranged and bonded, you may find it easier to remember their respective formulas.
What to Focus on for Multiple Choice

When preparing for multiple-choice questions, it’s essential to focus on key concepts and problem-solving strategies that are frequently tested. These types of questions typically assess your understanding of fundamental principles and your ability to apply them in various scenarios. The key is to concentrate on areas where you are most likely to encounter questions and refine your approach to answering them accurately and efficiently.
Understand Key Concepts
Multiple-choice questions often target core concepts such as formulas, classifications, and basic theories. Reviewing definitions, chemical symbols, and important equations can help you quickly identify correct answers. It’s also crucial to familiarize yourself with the types of questions that highlight differences between similar terms or concepts, which is a common strategy used in these questions.
Practice Problem-Solving Techniques
In addition to knowing the theory, practicing problems helps solidify your understanding. Focus on how to approach calculations, predictions, and comparisons. Practice by working through a variety of example questions, especially those that challenge you to apply concepts in new ways. This will not only help you recognize patterns but also improve your ability to narrow down options during the test.
How to Handle Experimental Questions
When faced with experimental questions, the key is to focus on understanding the procedure, the variables involved, and the expected outcomes. These types of questions assess your ability to apply theoretical knowledge to real-world scenarios, testing your understanding of scientific methods and reasoning. It’s essential to read each question carefully and approach it systematically to ensure you don’t miss any important details.
Start by identifying the purpose of the experiment or procedure described. Focus on the main steps of the process, the equipment used, and any specific conditions that might affect the results. Be sure to understand how different factors, such as temperature or concentration, influence the reactions or results. This will help you answer questions related to observations, calculations, or conclusions accurately.
Next, consider the type of data or measurements that would be collected in the experiment. This will help you determine the appropriate units, formulas, and calculations required to solve any related problems. Understanding the expected outcomes of the experiment also allows you to spot any discrepancies or errors in the data, which is a common element in experimental-based questions.
Understanding Thermochemistry Concepts
In this section, we delve into the study of energy changes that occur during chemical reactions and physical transformations. Thermochemical concepts are crucial in understanding how energy flows within a system, influencing the behavior of matter and the course of reactions. By studying these concepts, you’ll gain insights into the relationships between heat, work, and internal energy, essential for mastering various scientific principles and calculations.
One of the fundamental ideas in this field is the concept of heat transfer, where energy is either released or absorbed during a reaction. The law of conservation of energy states that energy cannot be created or destroyed, only transferred. This means that energy changes must always be accounted for in reactions, allowing for predictions about temperature changes, phase transitions, and reaction spontaneity.
Key Thermochemical Equations
Thermochemical equations provide valuable information about the energy involved in a process. They help quantify the amount of heat released or absorbed when a reaction occurs under standard conditions. Below is a simple representation of such an equation:
| Reaction | Enthalpy Change (ΔH) |
|---|---|
| H₂ + ½ O₂ → H₂O | -241.8 kJ/mol |
| C + O₂ → CO₂ | -393.5 kJ/mol |
Understanding Heat Capacity
Another essential concept is heat capacity, which refers to the amount of heat required to raise the temperature of a substance by a given amount. The heat capacity of a system is directly related to the amount of energy needed to change its temperature. The formula for calculating heat energy is:
q = mcΔT
Where:
- q = heat energy absorbed or released (in joules)
- m = mass of the substance (in grams)
- c = specific heat capacity (in J/g·°C)
- ΔT = change in temperature (in °C)
By applying these formulas, you can calculate the heat involved in a variety of reactions and physical processes. Understanding thermochemical concepts allows for accurate predictions and a deeper understanding of how substances interact with energy in different environments.
Real-Life Applications of Knowledge in Science
The principles of science are not limited to textbooks; they play a significant role in everyday life and are integral to many fields, ranging from healthcare to environmental conservation. The application of scientific concepts helps improve the quality of life, solve complex problems, and advance technology. This section explores the practical uses of scientific knowledge in various industries, demonstrating how it shapes the world around us.
From developing new medicines to creating sustainable energy solutions, understanding the fundamental laws of nature allows experts to address some of society’s most pressing challenges. The knowledge gained through rigorous study helps to innovate solutions that can positively impact human health, the environment, and industries worldwide.
Applications in Medicine
One of the most profound uses of scientific knowledge is in healthcare. It allows for the creation of life-saving medications, diagnostic tools, and treatment methods. For example:
- Drug Development: Understanding the molecular behavior of substances has led to the creation of pharmaceuticals that treat various diseases and infections.
- Diagnostic Techniques: Scientific advancements have improved the accuracy of tests and equipment, making it possible to detect illnesses early and treat them effectively.
- Medical Devices: The design of devices like pacemakers and insulin pumps relies on scientific principles to improve patient care and quality of life.
Environmental Impact and Sustainability
Science is also crucial in addressing environmental issues and promoting sustainability. Key areas of application include:
- Renewable Energy: The development of solar, wind, and hydroelectric power systems is based on scientific knowledge of energy and materials, providing cleaner alternatives to fossil fuels.
- Waste Management: Scientific techniques are used to recycle materials, reduce waste, and find sustainable ways to manage landfills.
- Climate Change Research: Understanding atmospheric science helps predict and mitigate the effects of global warming, leading to policy changes and new technologies aimed at reducing carbon footprints.
Technology and Innovation
Science is also foundational to technological advancement. From smartphones to computers, scientific principles power the innovation of devices and systems that define modern life:
- Electronics: Knowledge of electrical circuits, semiconductors, and materials science makes it possible to develop the devices we use daily, including computers, smartphones, and other high-tech gadgets.
- Artificial Intelligence: Algorithms and data processing techniques are based on mathematical and scientific models that allow machines to learn and adapt, driving advancements in AI.
- Space Exploration: The study of physics, chemistry, and engineering has enabled humans to explore outer space, developing technology to travel beyond our planet and study the universe.
The applications of scientific knowledge are far-reaching and continue to evolve. As we face new challenges and opportunities, this understanding remains key to finding innovative solutions that can enhance life on Earth and beyond.
How to Manage Time During Assessments
Effective time management is crucial when facing any assessment. With limited hours to demonstrate your understanding, it is important to strategize in order to maximize performance. By organizing tasks and setting priorities, you can navigate through the test efficiently and avoid unnecessary stress.
In this section, we will explore some key strategies to help manage time effectively during assessments, ensuring that every moment is used productively.
Pre-Assessment Preparation
Before entering the assessment room, taking a few simple steps can significantly improve your time management:
- Know the Format: Familiarize yourself with the structure of the test. Understanding whether it includes multiple choice, short answer, or essay questions can help you allocate your time more appropriately.
- Prioritize Study Areas: Focus on key concepts that are most likely to appear. Reviewing summaries, practice problems, or past assessments can help reinforce these areas.
- Get Enough Rest: Sleep is essential for concentration and focus. Being well-rested will help you think clearly and manage time more efficiently during the test.
During the Assessment
Once the assessment begins, managing your time wisely is essential. Here are some techniques to help you stay on track:
- Read Through the Entire Paper: Spend the first few minutes scanning the entire assessment to get an overview of the questions. This will allow you to identify which sections may require more time and which are quicker to answer.
- Divide Time Wisely: Set a time limit for each section based on the number of questions. If you have an hour and twenty minutes for 80 questions, for example, aim to spend around 1 minute per question in the multiple-choice section.
- Start with Easier Questions: Begin with questions that you are most confident about. This helps build momentum and saves time for more challenging sections later on.
- Stay on Track: Keep an eye on the clock without becoming distracted by it. If a question is taking too long, move on and come back to it later if time permits.
- Leave Time for Review: Reserve the last 10 minutes to review your answers. Check for any mistakes or incomplete responses, ensuring that everything is well-structured and coherent.
Additional Tips
Here are a few more tips to help you manage your time effectively:
- Practice Time-Based Mock Tests: Simulate test conditions by practicing with timed mock assessments. This helps you get accustomed to working within time limits.
- Stay Calm and Focused: Avoid panicking if you feel like you’re running out of time. Staying calm allows you to think more clearly and make better decisions under pressure.
- Use All Available Resources: If allowed, use any scratch paper or notes to organize your thoughts before answering complex questions. This can save time in the long run.
By incorporating these strategies into your approach, you can approach assessments with greater confidence and efficiency. Time management is a skill that, with practice, can significantly enhance your performance and reduce test anxiety.