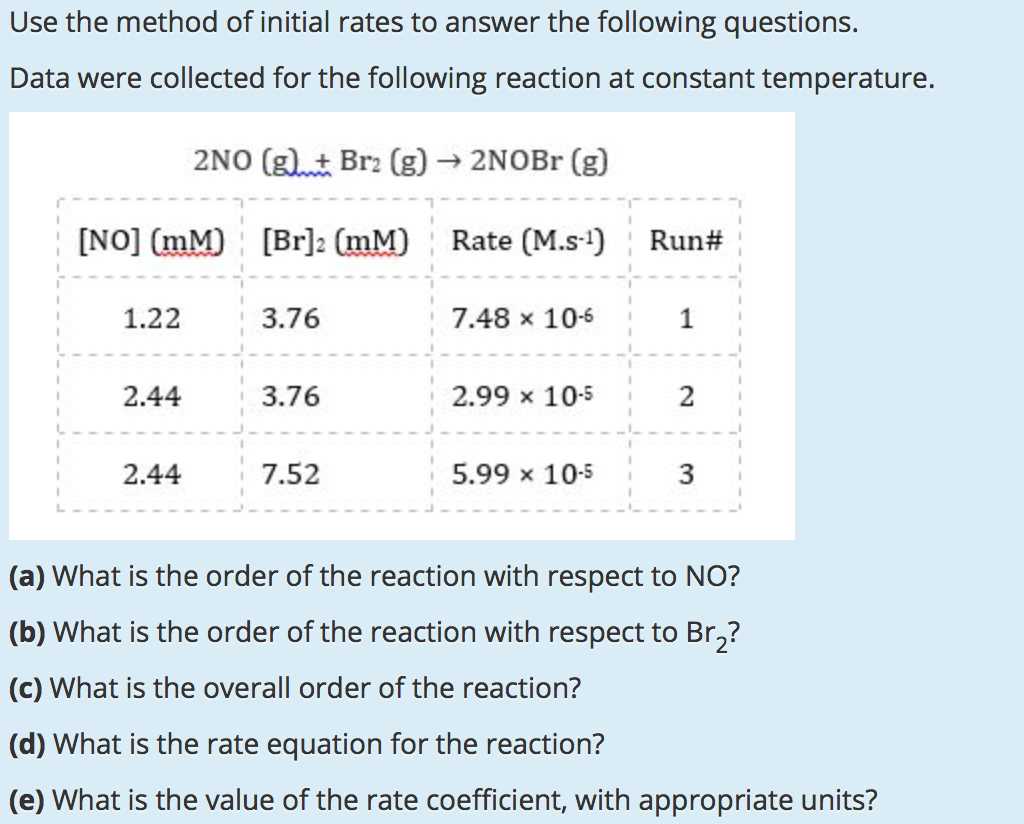
In chemistry, studying how reactions occur over time is essential to understanding their mechanisms. By analyzing how the speed of a reaction changes under different conditions, we gain valuable insights into the underlying processes. This approach helps in determining the factors that influence chemical transformations and the relationships between them.
One of the key tools used to explore this is examining how different variables, such as concentration or temperature, affect the speed of a reaction. Through structured learning techniques, students can develop a deeper understanding of these concepts. By carefully analyzing experimental data, they can uncover the fundamental principles governing reaction behavior.
Through practical exercises, learners can better comprehend how scientists predict reaction outcomes and optimize conditions for various industrial and laboratory applications. A systematic approach to analyzing experimental results is crucial for accurately interpreting chemical phenomena and advancing scientific knowledge.
Method of Initial Rates POGIL Answers
In chemical kinetics, understanding how the speed of a reaction is influenced by various factors is crucial for developing accurate models. By systematically analyzing experimental data, students can uncover relationships between different variables and their effects on the speed of chemical processes. This approach allows for the determination of reaction mechanisms and the calculation of important parameters such as the reaction order and rate constant.
Through carefully designed exercises, learners are introduced to techniques for interpreting experimental results. By manipulating concentrations or other reaction conditions, they can observe how these factors impact the progress of a reaction. These activities provide a hands-on opportunity to explore the underlying principles of reaction kinetics and develop problem-solving skills.
Accurate data analysis is central to these exercises, as it enables students to draw meaningful conclusions from observed patterns. Through this process, learners gain a deeper understanding of how scientists approach the study of chemical reactions and learn to apply these concepts to real-world scenarios. This method helps reinforce key principles and prepares students for more advanced studies in chemistry and related fields.
Understanding the Concept of Rate Laws
In chemical reactions, the speed at which reactants are converted into products depends on several factors. These factors can be expressed through mathematical equations that describe the relationship between the reaction rate and the concentrations of the reactants. Understanding these equations, known as rate laws, is fundamental to predicting how changes in conditions affect the speed of a reaction.
The Role of Concentration in Reaction Speed
Rate laws allow chemists to quantify how the concentration of reactants influences the reaction speed. For many reactions, an increase in the concentration of reactants leads to a higher reaction rate. This relationship can be described using an equation that includes the concentrations of the reactants raised to specific powers, known as the reaction orders.
| Reactant Concentration | Effect on Reaction Rate | Order of Reaction |
|---|---|---|
| Doubling concentration | Rate increases by a factor of 2 | First order |
| Doubling concentration | Rate increases by a factor of 4 | Second order |
| Doubling concentration | No change in rate | Zero order |
Interpreting the Rate Law Equation
The general form of a rate law equation is written as:
Rate = k[A]^m[B]^n,
where k is the rate constant, and [A] and [B] are the concentrations of reactants A and B. The exponents m and n are the reaction orders, which are determined experimentally. Understanding the values of these exponents helps scientists predict how the reaction will behave under different conditions.
How Initial Rates Help in Reactions
Understanding how quickly a reaction proceeds from the start provides valuable insights into the underlying mechanisms. By measuring how the speed of a reaction changes over time, scientists can identify factors that influence the process and determine the reaction’s characteristics. These observations are crucial for formulating models that describe chemical behavior and predicting how reactions will behave under different conditions.
One of the key advantages of studying the reaction speed at the beginning of a process is that it minimizes the influence of side reactions or changes that might occur as the reaction progresses. This initial data helps isolate the effect of specific variables, such as concentration or temperature, on the overall reaction speed.
- Reaction Order Determination: By analyzing the rate at the start, the order of the reaction with respect to each reactant can be determined.
- Reaction Mechanism Insight: Early-rate measurements help in proposing potential pathways for how reactants are converted into products.
- Rate Constant Calculation: Initial measurements allow for the determination of the rate constant, which is vital for predicting reaction behavior under various conditions.
- Effect of Concentration: Changes in the concentration of reactants at the beginning help identify the relationship between concentration and speed.
By focusing on the initial stage of the reaction, scientists can develop clearer models that predict reaction outcomes with greater accuracy. This approach forms the foundation for understanding more complex reactions and can guide practical applications in fields such as industrial chemistry and pharmaceuticals.
The Role of Concentration in Rates
The speed at which a chemical reaction occurs is influenced by several factors, one of the most significant being the concentration of the reactants. As the concentration of a reactant increases, the number of molecules or ions available for collision also increases, often leading to a faster reaction. Understanding this relationship helps scientists manipulate reaction conditions to achieve desired outcomes, whether in industrial processes or laboratory experiments.
How Concentration Affects Reaction Speed
For many reactions, the reaction speed increases when the concentration of the reactants is raised. This is because there are more molecules or ions in the system, which leads to more frequent collisions between reactant particles. However, the effect of concentration is not always linear, and it depends on the specific reaction and its order.
| Concentration Change | Effect on Reaction Speed | Example of Reaction Order |
|---|---|---|
| Doubling concentration | Rate increases by a factor of 2 | First-order reaction |
| Doubling concentration | Rate increases by a factor of 4 | Second-order reaction |
| Doubling concentration | No change in rate | Zero-order reaction |
Understanding Reaction Order
The effect of concentration on the speed of a reaction is often quantified by the reaction order, which refers to the power to which the concentration of a reactant is raised in the rate law. For example, in a first-order reaction, the speed is directly proportional to the concentration of one reactant. In a second-order reaction, the speed is proportional to the square of the concentration. Identifying the reaction order allows scientists to predict how changes in concentration will impact the speed of the reaction and helps in optimizing reaction conditions.
Exploring Reaction Orders with Examples
In chemical kinetics, the order of a reaction refers to the relationship between the concentration of reactants and the speed at which the reaction occurs. It plays a crucial role in understanding how different factors influence reaction mechanisms and how changes in reactant concentrations affect the overall process. By studying reaction orders, scientists can better predict reaction behavior and optimize experimental conditions.
The order of a reaction is determined experimentally and can vary depending on the reaction itself. It is expressed as an exponent in the rate law equation, which shows how the concentration of each reactant affects the overall speed. For example, a reaction can be zero-order, first-order, or second-order with respect to a specific reactant, and each of these types exhibits unique characteristics in how the reaction progresses.
Below are examples illustrating how reaction orders influence the speed of a reaction:
- Zero-Order Reaction: In a zero-order reaction, the speed is independent of the concentration of the reactant. Doubling the concentration does not affect the rate of the reaction.
- First-Order Reaction: In a first-order reaction, the rate is directly proportional to the concentration of one reactant. If the concentration of the reactant is doubled, the speed of the reaction will also double.
- Second-Order Reaction: For a second-order reaction, the rate is proportional to the square of the concentration of a reactant. Doubling the concentration increases the rate by a factor of four.
These examples demonstrate how understanding the order of a reaction allows scientists to predict how the reaction will behave under different conditions and provides essential information for designing experiments and optimizing processes in various fields, including industrial chemistry and pharmaceuticals.
Determining Rate Constants in Reactions
The rate constant is a fundamental parameter in chemical kinetics that provides insight into the speed of a reaction under specific conditions. It is a proportionality factor in the rate law equation that links the concentration of reactants to the rate of the reaction. Determining this constant is essential for understanding how a reaction will behave at different concentrations and temperatures. Its value is unique to each reaction and can be affected by various factors such as temperature and the presence of catalysts.
Methods for Determining the Rate Constant
There are several ways to determine the rate constant, each depending on the type of reaction and available experimental data. One common approach is to use experimental measurements of the reaction rate at different concentrations of reactants. By plotting the data and analyzing the graph, the rate constant can be calculated. The most straightforward method often involves applying the integrated form of the rate law for specific reaction orders, such as first-order or second-order reactions.
Using Experimental Data to Calculate k
For a reaction with a known order, the rate constant k can be determined by measuring how the concentration of reactants changes over time. For example, in a first-order reaction, the rate law is expressed as:
Rate = k[A]
If the concentration of reactant A is measured at different time intervals, a plot of ln[A] versus time will yield a straight line, and the slope of this line is equal to -k. Similarly, for a second-order reaction, a plot of 1/[A] versus time will yield a line, and the slope will correspond to k.
Once the rate constant is determined experimentally, it can be used to predict reaction behavior under different conditions, making it an essential tool for chemists and engineers working with chemical processes.
Using POGIL to Analyze Reaction Mechanisms
In chemical kinetics, understanding the sequence of steps that lead from reactants to products is essential for predicting and controlling reactions. By breaking down a complex reaction into smaller, sequential steps, researchers can better understand the underlying mechanisms. One effective way to approach this analysis is through guided inquiry-based learning techniques, which encourage critical thinking and collaboration. This method helps students and scientists alike explore how different factors influence reaction pathways and how to deduce the mechanisms from experimental data.
Steps to Analyze Reaction Mechanisms
When analyzing reaction mechanisms, there are several key steps involved. The process typically starts with formulating a hypothesis about the sequence of events that occurs during the reaction. Then, experimental data is collected to validate or refine this hypothesis. By examining the relationship between reactant concentrations, time, and the observed changes, scientists can infer the individual steps involved in the overall process.
- Step 1: Identify the overall reaction and its components.
- Step 2: Break the reaction down into elementary steps.
- Step 3: Determine the rate law for each step.
- Step 4: Use experimental data to refine and validate the proposed mechanism.
- Step 5: Correlate the results with the proposed model and adjust as necessary.
Collaboration and Inquiry-Based Learning
Guided inquiry learning techniques are valuable tools in teaching and studying reaction mechanisms. By working in groups and applying a step-by-step approach, individuals can share insights and challenge assumptions. This process not only enhances understanding but also fosters a deeper connection to the material. Through this collaborative effort, students and researchers can explore the complexity of reaction mechanisms and apply their findings to real-world scenarios, such as designing efficient chemical processes or developing new catalysts.
Step-by-Step Guide to Rate Experiments
Conducting experiments to determine how quickly a reaction occurs involves carefully measuring various factors that influence the speed of the process. Understanding how different variables, such as concentration, temperature, and the presence of catalysts, affect the reaction is crucial for drawing accurate conclusions. This guide provides a clear, systematic approach for designing and performing experiments to study the dynamics of chemical reactions.
Each experiment requires precise measurements and controlled conditions to ensure reliable results. By following a step-by-step procedure, one can gather the necessary data to analyze how changes in specific factors influence the reaction’s speed. The process involves setting up the reaction, recording measurements at various time intervals, and analyzing the data to determine the relationship between the variables.
Step 1: Prepare the Reaction
Begin by selecting the appropriate reactants and ensuring that the necessary equipment is available. Be sure to use the correct concentrations for the reactants and set up the experiment in a controlled environment to minimize external factors. The goal is to ensure that the reaction occurs under the desired conditions, which will allow for accurate measurement of the reaction speed.
Step 2: Measure Key Variables
During the experiment, closely monitor the changes in concentration or other variables at regular intervals. This could involve measuring the amount of product formed, the time taken for a particular color change, or any other observable physical change. Be sure to record these observations carefully to ensure the accuracy of the data.
Step 3: Analyze the Data
Once data has been collected, analyze it to determine the relationship between the factors being tested and the reaction speed. Plotting the data on graphs can often reveal trends, such as linear or exponential relationships, that help identify how different variables influence the reaction. By calculating slopes or other relevant metrics, you can derive useful conclusions about the reaction mechanism and its dependencies.
By following this structured approach, researchers can gain valuable insights into the factors that govern reaction behavior, making it possible to optimize conditions for various chemical processes and applications.
Graphical Methods for Initial Rate Analysis
In chemical kinetics, graphical techniques are valuable tools for visualizing and analyzing the relationship between the concentration of reactants and the speed of a reaction. By plotting data in different ways, scientists can more easily identify trends and determine key parameters, such as reaction order and rate constants. These visual methods simplify the process of interpreting experimental results and help in deriving important kinetic information.
Using Concentration vs. Time Graphs
One of the most common graphical methods involves plotting the concentration of a reactant or product over time. This type of graph can reveal how the concentration changes as the reaction progresses and is particularly useful for determining the reaction’s order. For example, in a first-order reaction, a plot of the natural logarithm of the concentration versus time yields a straight line, from which the rate constant can be calculated. Similarly, for second-order reactions, a plot of the inverse concentration versus time will yield a straight line.
Logarithmic Plots and Their Applications
Another important graphical approach involves plotting the logarithm of the concentration against time. This method is particularly useful when determining the order of a reaction. By comparing different plots–such as ln[A] for first-order or 1/[A] for second-order reactions–researchers can quickly assess which type of plot yields a linear relationship. This linearity can then be used to deduce the reaction’s order and, in turn, calculate the rate constant.
These graphical methods simplify the analysis of experimental data and provide a clear visual representation of how different factors affect the reaction. By applying these techniques, chemists can gain a deeper understanding of reaction mechanisms and optimize conditions for various chemical processes.
Common Mistakes in Initial Rate Experiments
In experimental chemistry, even small errors in the setup or procedure can lead to significant inaccuracies in the results. Understanding the most common mistakes that can occur during the process of measuring the speed of a reaction helps in ensuring reliable data collection. By recognizing these issues, researchers can improve the accuracy of their experiments and avoid misleading conclusions.
Poor Measurement of Concentrations
One of the most frequent errors in experiments is improper measurement of the concentrations of reactants. If the concentrations are not accurately determined, it can lead to incorrect assumptions about the reaction’s order and rate constant. This mistake can occur due to poor calibration of equipment or incorrect handling of substances. To avoid this, ensure that all reagents are prepared with precise measurements, and double-check the concentration values before starting the experiment.
Inconsistent Time Intervals
Another common mistake is the use of inconsistent or inadequate time intervals when recording data. If time measurements are not taken at regular intervals, it becomes difficult to analyze how the reaction progresses. The timing of measurements should be consistent throughout the experiment, as irregular intervals can lead to errors in determining the relationship between concentration and time. Using automated data collection systems or timers can help eliminate this issue.
| Error Type | Consequences | Prevention |
|---|---|---|
| Poor concentration measurement | Incorrect rate law, inaccurate calculations | Carefully measure and double-check concentrations |
| Inconsistent time intervals | Difficulty in determining reaction order and rate constant | Use consistent, regular time intervals |
| Temperature fluctuations | Inaccurate reaction rate data | Control and monitor the temperature carefully |
Temperature Fluctuations
Temperature plays a crucial role in reaction rates. Fluctuations in temperature during the experiment can lead to inaccurate results, as even slight changes can significantly affect the speed of the reaction. To minimize this issue, it is essential to control the temperature throughout the experiment and use temperature-regulated equipment. This ensures that all data reflects the intended experimental conditions and that reaction rates are measured under stable circumstances.
By avoiding these common mistakes, researchers can ensure that their experiments provide reliable and accurate data, ultimately leading to better insights into chemical reaction dynamics.
Understanding the Impact of Temperature on Rates
Temperature is one of the most influential factors affecting the speed of chemical reactions. As temperature increases, the kinetic energy of molecules also rises, which in turn accelerates the reaction process. This relationship is crucial to understand, as even small changes in temperature can significantly alter the outcome of an experiment. By exploring how temperature affects reaction speed, scientists can better control and optimize reaction conditions for desired results.
The Kinetic Theory and Temperature
According to the kinetic molecular theory, the temperature of a system is directly proportional to the average kinetic energy of its particles. As molecules move faster at higher temperatures, they collide more frequently and with greater energy, increasing the chances of overcoming the activation energy barrier. This leads to a faster reaction rate. Conversely, at lower temperatures, molecules move slower, resulting in fewer successful collisions and a slower reaction.
Arrhenius Equation and Temperature Dependence
The Arrhenius equation provides a mathematical expression for the temperature dependence of reaction rates. This equation describes how the rate constant changes with temperature, highlighting the exponential relationship between these two factors. As the temperature increases, the rate constant typically increases, leading to a faster reaction. Understanding this equation is key in predicting how temperature changes will affect a chemical reaction.
Practical Considerations in Temperature Control
- Ensure consistent temperature conditions throughout the experiment to avoid fluctuations that could skew results.
- Use temperature-controlled equipment, such as water baths or thermostats, to maintain stable reaction conditions.
- Monitor the temperature at regular intervals to track its effect on the reaction progress.
By taking these factors into account, researchers can gain more accurate data and better understand the role temperature plays in chemical kinetics. Whether optimizing industrial processes or conducting laboratory experiments, controlling temperature is essential for achieving reliable and reproducible results.
Comparing Rate Laws for Different Reactions
Understanding how different reactions behave under varying conditions is essential in chemical kinetics. By analyzing and comparing how the speed of various reactions changes with respect to the concentrations of reactants, we can derive distinct mathematical expressions that describe their behavior. These expressions, often referred to as the rate laws, provide a framework for understanding the underlying mechanisms of reactions, allowing scientists to predict how different factors influence the progress of a chemical process.
Types of Reactions and Their Rate Laws
Different chemical reactions exhibit different behaviors depending on their molecular mechanisms. Some reactions may depend on the concentration of one reactant, while others may be influenced by multiple reactants. The rate law for each reaction reflects these dependencies and can be classified as follows:
- Zero-Order Reactions: The reaction rate remains constant, unaffected by the concentration of the reactants.
- First-Order Reactions: The rate is directly proportional to the concentration of one reactant.
- Second-Order Reactions: The rate is proportional to the square of the concentration of one reactant or to the product of the concentrations of two reactants.
- Complex Reactions: These reactions may involve multiple reactants, and the rate law is derived from experimental data, often requiring a detailed understanding of the reaction mechanism.
Experimental Methods for Comparing Rate Laws
To compare the rate laws of different reactions, experiments must be carefully designed to measure how the reaction rate changes with varying concentrations of reactants. The following methods are commonly used:
- Method of Isolation: One reactant is kept in excess while the concentration of the other reactant is varied, allowing for a simplified analysis of how one reactant affects the rate.
- Integrated Rate Laws: By measuring how the concentration of reactants changes over time, integrated rate laws can be derived to describe the reaction’s progress.
- Graphical Methods: Plotting concentration versus time data for different reactions can help visualize and compare reaction orders based on the shape of the resulting curves.
Once the rate laws for each reaction are determined, scientists can compare them to identify patterns and understand how different factors–such as temperature, catalysts, or solvent conditions–affect the speed of the reaction. This comparison can provide deeper insights into the mechanisms that govern the transformation of reactants into products.
POGIL Approach to Teaching Kinetics
The POGIL approach, which stands for Process Oriented Guided Inquiry Learning, offers a unique and effective way to teach complex concepts in chemistry, including the study of how reactions occur and progress. Unlike traditional lecture-based methods, this strategy emphasizes student-centered learning, where students actively engage in exploring and solving problems through guided inquiry. By applying this approach to kinetics, students are encouraged to construct their own understanding of reaction mechanisms and the factors that influence reaction speeds.
Active Learning Through Guided Exploration
In a POGIL classroom, students work in small groups to investigate a series of questions designed to guide their thinking and facilitate discovery. The process involves analyzing experimental data, formulating hypotheses, and testing these ideas through discussion and collaboration. This hands-on, collaborative method fosters a deeper understanding of kinetic principles, as students are able to apply what they learn in a practical context, often leading to more meaningful insights.
Benefits of the POGIL Approach for Kinetics
Using this approach to teach kinetics offers several advantages:
- Increased Engagement: Students are actively involved in their learning, which promotes better retention and understanding of the material.
- Critical Thinking Development: By solving problems and analyzing data, students improve their ability to think critically and apply concepts to new situations.
- Collaboration and Communication: Working in groups helps students develop teamwork and communication skills, essential for scientific inquiry.
- Deeper Conceptual Understanding: The inquiry-based format allows students to explore the underlying principles of kinetics in a way that textbooks alone cannot provide.
Overall, the POGIL approach empowers students to take ownership of their learning, helping them to not only understand reaction rates and mechanisms but also to develop essential skills for scientific investigation.
Real-World Applications of Rate Laws
The study of how reactions occur and progress plays a crucial role in many aspects of our everyday lives. Understanding the factors that influence the speed of chemical processes is not only essential in academic research but also has far-reaching applications in various industries. From environmental science to pharmaceuticals, the principles governing reaction speeds are utilized to solve real-world problems and optimize processes in numerous fields.
Applications in Industry
One of the most prominent applications of reaction dynamics is in the manufacturing sector, where chemical reactions are often integral to product development. By understanding how reaction speed changes under different conditions, industries can:
- Optimize production rates: Ensuring the most efficient use of raw materials and energy in chemical manufacturing processes.
- Improve product quality: Fine-tuning reaction conditions to achieve desired outcomes, such as purity or consistency.
- Increase safety: Identifying potential hazards related to reaction speeds and implementing controls to prevent accidents.
Environmental Applications
In the realm of environmental science, understanding how pollutants degrade or transform in the environment is essential. By applying the principles of reaction kinetics, scientists can:
- Model pollutant breakdown: Predicting the speed at which contaminants degrade in air, water, or soil.
- Design better waste treatment processes: Developing more efficient ways to neutralize hazardous substances before they harm ecosystems.
- Assess environmental impact: Understanding how different environmental factors, such as temperature or pH, affect the rate of degradation of harmful substances.
Pharmaceutical Applications
In pharmaceuticals, the rate at which reactions occur is critical in the development of new drugs and treatments. Scientists use reaction kinetics to:
- Determine drug efficacy: Studying how quickly a drug is metabolized or interacts with the body.
- Control drug formulation: Ensuring that active ingredients react at the right speed to achieve the desired therapeutic effect.
- Develop sustainable manufacturing methods: Optimizing drug production processes to improve yield and reduce costs.
From industrial to environmental and pharmaceutical applications, the understanding of chemical reaction principles has vast practical implications. By applying these concepts, scientists and engineers can develop more efficient, safe, and environmentally friendly solutions to meet the demands of modern society.
How POGIL Enhances Chemistry Learning
Active learning strategies have proven to be highly effective in engaging students and enhancing their understanding of complex subjects like chemistry. By moving away from traditional lecture-based teaching and encouraging students to explore concepts through hands-on activities, this approach fosters deeper comprehension and critical thinking skills. The focus is on student-centered learning, where individuals actively participate in their own educational journey by collaborating, analyzing, and solving problems together.
Encouraging Collaborative Learning
One of the core aspects of this teaching method is its emphasis on teamwork. Students are often placed in small groups, where they can discuss and work through problems collectively. This collaborative learning environment helps students:
- Enhance problem-solving skills: By engaging in group discussions, students gain different perspectives and develop a more thorough understanding of concepts.
- Improve communication abilities: Working in teams teaches students how to explain their reasoning and articulate their thoughts clearly.
- Develop interpersonal skills: Collaborating with peers encourages mutual respect and the sharing of ideas, fostering a positive learning community.
Promoting Critical Thinking and Application
This approach challenges students to think critically about the concepts they encounter. Rather than passively receiving information, they are prompted to analyze data, identify patterns, and make connections between different pieces of knowledge. This strengthens their ability to apply theoretical knowledge to real-world situations. Students are encouraged to:
- Make connections: Relating abstract concepts to everyday examples helps reinforce learning and make chemistry more accessible.
- Ask probing questions: Rather than just memorizing facts, students are taught to question the material and explore deeper explanations.
- Apply knowledge creatively: The focus on application enables students to approach problems from various angles and come up with innovative solutions.
By shifting the focus from traditional passive learning to an active, collaborative, and inquiry-driven environment, this approach helps students develop not only a stronger understanding of chemistry but also the skills they need to succeed in real-world scientific endeavors. This transformative learning model prepares students for both academic and professional challenges by cultivating critical thinking, teamwork, and problem-solving abilities.
Tips for Solving Rate Law Problems Efficiently
When tackling problems related to the mathematical description of reaction speeds, it’s important to approach them methodically. These types of exercises can seem overwhelming at first, but by following a series of strategic steps, you can simplify complex scenarios and solve them more efficiently. The key is understanding the relationships between reactant concentrations and how they influence the speed of a chemical reaction. By organizing your work and applying logical reasoning, you can make the process more straightforward.
Break Down the Information Carefully
Before diving into calculations, make sure you clearly understand the problem. Break down the given data and ensure you are aware of all the variables involved. For example:
- Identify the given concentrations: Pay attention to which concentrations are provided and make sure to note whether they refer to reactants or products.
- Understand the experimental setup: Check if any specific conditions, such as temperature or pressure, are mentioned that could affect the calculations.
- Look for patterns: Often, problems will give you data points that exhibit a predictable trend. Identifying these patterns early on can help you recognize the order of the reaction or how the concentration influences the speed.
Use Logical Steps for Determining the Exponent
One of the most critical steps in these problems is determining the relationship between concentration and reaction speed. To do this efficiently:
- Compare data points: Look at multiple sets of data where concentration and speed are provided. Determine how changes in concentration affect the speed to calculate the exponent in the rate law.
- Use ratios: Ratios of concentration changes can help you isolate the exponent. By dividing one data set by another, you can find out how much the speed changes relative to concentration adjustments.
- Verify the consistency: After determining the order of the reaction, double-check that it holds true for all sets of data provided in the problem.
By following these techniques, you can approach rate law problems with confidence and efficiency. The more practice you get, the more intuitive it will become to recognize key patterns and relationships in the data, enabling you to solve problems with greater ease.