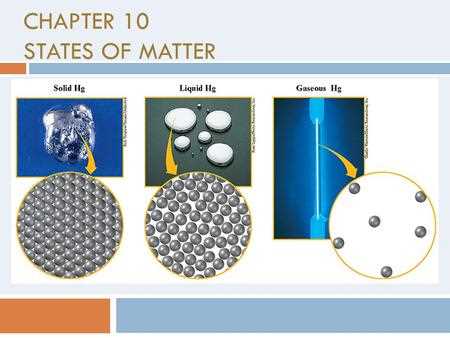
In this section, we explore how different forms of matter respond under various conditions. From solid to liquid to gas, substances exhibit unique characteristics that define their structure and function. The way these forms interact with changes in temperature, pressure, and energy is fundamental to a wide range of scientific studies.
Phase transitions play a crucial role in many natural and industrial processes. By understanding how substances shift between different forms, we can better explain their behavior in real-world situations. This knowledge is essential not only for theoretical applications but also for practical innovations in fields such as engineering and environmental science.
Energy exchanges during these transitions are key to understanding how materials react to external influences. These reactions help scientists and engineers manipulate matter for a variety of uses, from everyday products to complex technological systems.
Review of Key Concepts in Phase Transitions
This section delves into the essential ideas behind the behavior of different substances under varying conditions. Understanding the characteristics of solids, liquids, and gases allows us to explain a wide range of phenomena that occur in nature and technology. The principles governing the transformations between these forms provide the foundation for many scientific applications.
Exploring Energy and Temperature Effects
Temperature plays a crucial role in altering the arrangement and movement of molecules. When substances undergo heating or cooling, energy is either absorbed or released, influencing their physical state. These changes can be analyzed through the lens of energy exchange and molecular motion, which provides a deeper understanding of their behavior at the atomic level.
The Role of Pressure in Transitions
Pressure also influences the behavior of substances, particularly in gases. Changes in external pressure can trigger transitions from one phase to another, such as the boiling or freezing of liquids. This relationship between pressure and phase change is vital for comprehending both natural processes and engineered systems, such as those found in thermodynamics and material science.
| Phase | Energy Required | Typical Process |
|---|---|---|
| Solid to Liquid | Melting | Heat absorbed |
| Liquid to Gas | Vaporization | Heat absorbed |
| Gas to Liquid | Condensation | Heat released |
| Liquid to Solid | Freezing | Heat released |
Understanding the Forms of Matter
Substances exist in different forms depending on temperature, pressure, and other environmental factors. These forms exhibit unique properties that affect how they behave and interact with their surroundings. By studying these distinct phases, we can predict and explain how materials respond to changes in their environment, which is essential for both scientific research and practical applications.
Solids and Their Properties
In the solid form, particles are closely packed and move only slightly, which gives the substance a fixed shape and volume. The strong intermolecular forces hold the particles together, making solids rigid and stable. This is the reason why objects like metals, ice, and rocks maintain their shape under normal conditions.
Gases and Their Behavior
Unlike solids, gas particles are spread far apart and move freely, allowing gases to expand and fill any container. The low intermolecular forces between gas particles make them highly compressible and easily influenced by changes in temperature and pressure. This explains why gases such as oxygen, carbon dioxide, and nitrogen behave differently than liquids or solids.
Solid State Properties and Behavior
The solid form of a substance is characterized by tightly packed particles that are not free to move but instead vibrate in place. This unique arrangement results in specific properties that distinguish solids from liquids and gases. The strength of these properties depends largely on the forces between particles, which vary depending on the material in question. Understanding how solids behave under different conditions is fundamental to various scientific and industrial fields.
Key Characteristics of Solids
- Definite Shape and Volume: Solids maintain a consistent shape and volume due to the rigid arrangement of their particles.
- High Density: The particles are tightly packed, leading to higher density compared to liquids and gases.
- Low Compressibility: Solids resist compression because their particles are already in close contact.
- Strong Intermolecular Forces: The interactions between particles in a solid are stronger, giving it stability and structure.
Behavior of Solids Under External Conditions
When solids are exposed to temperature changes or pressure, their properties can shift. For example:
- Heating: As temperature increases, the particles vibrate more vigorously, which can cause the solid to expand or even transition to a liquid phase if enough heat is applied.
- Pressure: High pressure can force particles closer together, affecting the material’s density and in some cases, changing its structural properties.
These characteristics are essential for designing materials for specific applications, such as construction, manufacturing, and technology. By manipulating the properties of solids, scientists and engineers can develop stronger, more durable substances suited to a wide range of uses.
Characteristics of Liquids in Science
Liquids exhibit a unique set of properties that distinguish them from both solids and gases. In this phase, the particles are more loosely connected than in solids, allowing them to flow and take the shape of their container while maintaining a constant volume. The balance between attractive forces and kinetic energy governs the behavior of liquids, influencing their response to changes in temperature and pressure.
The properties of liquids are essential for understanding a variety of processes, from biological systems to industrial applications. These characteristics also play a significant role in phenomena such as fluid dynamics, heat transfer, and material science.
| Property | Explanation |
|---|---|
| Definite Volume | Liquids have a fixed volume, unlike gases, but can change shape depending on the container. |
| Fluidity | Liquids can flow and adapt to the shape of their surroundings due to the relatively free movement of particles. |
| Surface Tension | Attractive forces between molecules create a “skin” at the surface of liquids, allowing them to resist external force. |
| Viscosity | Liquids resist flow to varying degrees; higher viscosity means thicker, slower movement (e.g., honey vs. water). |
| Incompressibility | Liquids cannot be compressed easily, as the particles are already closely packed together. |
Gas Laws and Molecular Movement
The behavior of gases is governed by a set of principles that describe how particles move and interact under different conditions. These principles explain how gases expand to fill their containers, respond to changes in temperature, and how their pressure is influenced by volume and temperature. By understanding these laws, we can predict how gases behave in various environments, from industrial applications to natural phenomena.
Key Gas Laws
- Boyle’s Law: This law states that the pressure of a gas is inversely proportional to its volume when temperature is held constant. As the volume decreases, the pressure increases, and vice versa.
- Charles’s Law: This law asserts that the volume of a gas is directly proportional to its temperature when pressure is constant. As temperature rises, the gas expands.
- Avogadro’s Law: It states that the volume of a gas is directly proportional to the number of gas molecules, assuming temperature and pressure remain constant.
- Ideal Gas Law: The ideal gas law combines the previous laws into one equation, stating that pressure, volume, and temperature are related through the equation PV = nRT.
Molecular Movement in Gases
In the gaseous phase, particles are in constant, random motion. Their speed and energy depend on temperature, with higher temperatures resulting in faster movement. Gas molecules collide with each other and with the walls of their container, and these collisions determine the pressure exerted by the gas. The behavior of gas molecules can be described in terms of:
- Elastic Collisions: Gas particles collide without losing energy, meaning that the total kinetic energy of the system remains constant.
- Random Motion: Gas particles move in all directions and at varying speeds, which is why gases expand to fill any space available.
- Kinetic Energy: The average kinetic energy of gas molecules is directly proportional to the temperature of the gas.
By understanding these principles, we gain valuable insights into how gases behave in different environments, from air pressure in weather systems to the behavior of gases in engines or industrial processes.
Phase Transitions in Matter Explained
The process by which a substance changes from one form to another is a fundamental concept in science. These transformations occur when external conditions like temperature or pressure are altered, causing particles to rearrange and transition between different phases. Understanding these transitions is essential for explaining a variety of natural and industrial processes, from the freezing of water to the vaporization of liquid fuels.
Each transition is driven by changes in the energy of the system. When energy is added, particles gain movement and may overcome the forces holding them in place, leading to a transition to a higher-energy phase. Conversely, removing energy can cause particles to slow down and settle into a lower-energy state. These changes are not only crucial in everyday phenomena but also play a significant role in applications ranging from material design to environmental science.
Temperature’s Effect on Matter Forms
Temperature plays a crucial role in determining how substances behave and transform between different forms. As heat is added or removed, the energy of particles increases or decreases, leading to changes in their arrangement and movement. These alterations in energy can cause a substance to shift from one phase to another, such as from solid to liquid or liquid to gas. Understanding this relationship is fundamental to explaining everyday phenomena and scientific processes.
Increased heat causes particles to move more rapidly, often overcoming the forces that hold them in their current phase. Conversely, lowering the temperature slows down particle movement, causing them to settle into a more ordered structure. This interaction between energy and particle behavior governs the transitions and stability of different forms, influencing everything from the boiling of water to the freezing of liquids in colder environments.
Pressure’s Role in Phase Changes
Pressure has a significant impact on the transformation of substances from one form to another. When the external pressure on a substance is altered, it can either promote or hinder the ability of particles to change phases. This influence is particularly noticeable in the processes of melting, boiling, and sublimation. By controlling pressure, it is possible to manipulate the conditions under which these transformations occur, which is essential in both natural processes and industrial applications.
Increasing pressure typically forces particles closer together, which can cause a substance to shift to a more compact phase, such as turning a gas into a liquid. On the other hand, decreasing pressure allows particles to spread out, often leading to the opposite effect, such as liquid boiling at lower temperatures. This relationship between pressure and phase behavior is crucial in understanding everything from weather systems to the design of pressure cookers.
In summary, pressure plays a key role in controlling the behavior of substances under various conditions. Whether it’s raising the boiling point of water or preventing ice from melting, the manipulation of pressure allows for precise control over phase changes in a variety of contexts.
Energy Changes During State Transitions
During phase transitions, energy is either absorbed or released by a substance as it shifts from one form to another. These energy changes are essential for understanding how and why materials change their behavior in response to alterations in temperature or pressure. When a substance moves from one phase to another, the particles either gain or lose energy, which directly affects their movement and arrangement.
In some transitions, energy is absorbed, which allows particles to overcome the forces holding them together, while in others, energy is released as particles become more tightly packed. These processes are key to a variety of natural and industrial processes, from the melting of ice to the condensation of steam. Understanding how energy influences these transitions is fundamental in fields such as material science, engineering, and environmental science.
Energy Absorption vs. Energy Release
| Phase Transition | Energy Change |
|---|---|
| Melting | Energy is absorbed as a solid turns into a liquid. |
| Freezing | Energy is released as a liquid turns into a solid. |
| Vaporization | Energy is absorbed as a liquid turns into a gas. |
| Condensation | Energy is released as a gas turns into a liquid. |
| Sublimation | Energy is absorbed as a solid turns directly into a gas. |
| Deposition | Energy is released as a gas turns directly into a solid. |
Critical Point and Its Importance
The critical point marks a distinct condition at which the boundary between liquid and gas phases disappears, and the substance enters a unique state. At this point, the substance can no longer be distinguished as either liquid or gas, as both phases merge into a single, homogeneous phase. This phenomenon is crucial in understanding the behavior of substances under extreme conditions, where traditional phase transitions no longer apply.
Understanding the critical point is vital for numerous scientific and industrial applications, such as in the production of supercritical fluids, which have properties of both liquids and gases. These fluids are used in processes like extraction, cleaning, and even as solvents. The ability to manipulate substances near their critical point allows for greater control in various technologies, making this concept essential in fields ranging from materials science to environmental engineering.
In summary, the critical point represents a unique phase in the behavior of substances, marking a threshold beyond which their properties undergo significant changes. Understanding its significance helps scientists and engineers harness the potential of materials under extreme conditions, opening doors to innovative applications.
Exploring the Kinetic Molecular Theory
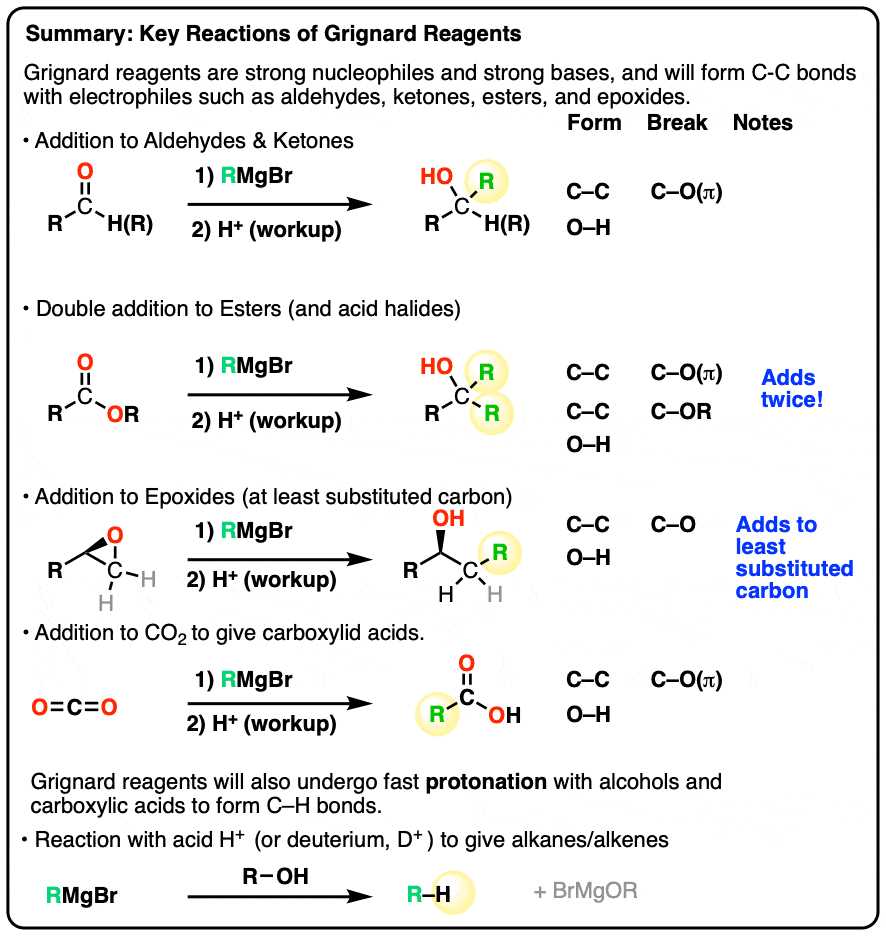
The Kinetic Molecular Theory provides a framework for understanding the behavior of particles in different forms of matter. It explains how the movement and interactions of particles dictate the physical properties of solids, liquids, and gases. According to this theory, particles are in constant motion, and their energy determines the state and behavior of the substance. This theory plays a crucial role in predicting and explaining phenomena such as temperature changes, pressure variations, and phase transitions.
Key principles of the Kinetic Molecular Theory suggest that the motion of particles is influenced by both their temperature and the forces between them. For example, in a gas, particles move freely and rapidly, while in a solid, the particles are tightly packed and vibrate in place. The theory also helps explain why gases expand to fill their containers or why liquids maintain a constant volume while flowing.
Ultimately, this theory allows scientists to model and predict the behavior of materials under varying conditions, providing valuable insights into everything from the design of new materials to the understanding of natural processes like weather and atmospheric changes.
Viscosity and Its Impact on Liquids

Viscosity refers to the resistance of a liquid to flow. It is a measure of how thick or sticky a liquid is and how easily it can move. This property plays a critical role in various natural and industrial processes, influencing everything from the flow of water in rivers to the production of oils and syrups. Liquids with high viscosity flow slowly, while those with low viscosity flow more easily.
The behavior of liquids in motion is significantly affected by their viscosity. For instance, in cooking or manufacturing, understanding viscosity helps determine the best methods for mixing, pouring, or pumping liquids. In the natural world, it also impacts how substances like lava or honey behave under different conditions. Temperature and molecular structure are key factors that affect the viscosity of a liquid–warmer liquids generally have lower viscosity, meaning they flow more freely, while cooler liquids tend to become thicker.
In summary, viscosity is a fundamental property of liquids that affects their movement and interaction with other substances. It has practical applications across a wide range of industries, from food production to automotive engineering, making it essential for both scientific study and everyday activities.
Density and Behavior of Gases
The density of a gas plays a crucial role in determining its behavior and properties under various conditions. Unlike solids and liquids, gases have low density due to the wide spacing between their particles. This low density causes gases to expand and fill any container, a characteristic that is essential for understanding how gases interact with their environment and with other substances. The relationship between temperature, pressure, and volume is key to predicting how a gas will behave under changing conditions.
As temperature increases, the particles in a gas move faster, and the gas tends to expand, leading to a decrease in density. Conversely, when the temperature decreases, the gas particles slow down, and the gas contracts, increasing its density. Additionally, the pressure applied to a gas can compress it, leading to a higher density. The interplay between these factors is governed by gas laws, such as Boyle’s law and Charles’ law, which describe how gases respond to changes in volume, pressure, and temperature.
Understanding the density of gases is essential in various applications, from atmospheric science to industrial processes. It helps in the design of gas storage systems, the calculation of gas flow rates, and the analysis of gas mixtures. By manipulating conditions such as pressure and temperature, we can control the behavior of gases, making this concept a fundamental part of many scientific and engineering fields.
Changes in Matter During Heating
When heat is applied to substances, their physical and sometimes chemical properties can change. These changes occur because the energy from the heat affects the movement of particles within the substance. Depending on the amount of heat and the substance’s characteristics, different transformations can take place, including changes in phase, volume, or even molecular structure.
Phase Transitions
Heating can cause a substance to transition between different phases, such as from solid to liquid or from liquid to gas. These transitions involve the breaking or formation of bonds between particles. For example:
- Melting: Solid turns to liquid as the heat overcomes the forces holding the particles in place.
- Boiling: Liquid turns to gas as heat gives particles enough energy to escape from the liquid phase.
- Sublimation: Solid turns directly into gas without passing through the liquid phase (e.g., dry ice).
Other Changes in Properties
Besides phase transitions, heating can also cause changes in other properties such as volume and density:
- Thermal expansion: As a substance is heated, its particles move faster and tend to spread apart, causing the substance to expand in volume.
- Density: As the substance expands, its density decreases since the particles are spread further apart.
Understanding how substances change during heating is essential in various fields, such as cooking, material science, and even environmental studies, as it helps predict how different substances will behave under different thermal conditions.
Equilibrium in Multiple Phases
When a substance exists in more than one phase, the interactions between these phases can lead to a state of balance, where the processes affecting the substance in each phase occur at equal rates. This balance, known as equilibrium, is crucial in understanding how substances transition between phases, and how these transitions can occur simultaneously in different parts of the system. In a system with multiple phases, the substance may continuously shift between solid, liquid, and gaseous forms, but at equilibrium, the rates of these changes remain constant over time.
Dynamic Nature of Equilibrium
At equilibrium, the processes occurring in different phases do not cease; rather, they balance out. For example:
- Evaporation and Condensation: In a closed container, some liquid molecules evaporate into the gas phase, while others condense back into the liquid phase. At equilibrium, the rate of evaporation equals the rate of condensation.
- Melting and Freezing: In a system where solid and liquid forms coexist, melting and freezing happen simultaneously. The rate of melting equals the rate of freezing when equilibrium is reached.
Factors Affecting Equilibrium
The balance between phases can be influenced by several factors, including temperature and pressure. Changes in these variables can shift the equilibrium point, leading to a higher concentration of one phase over another. Key factors include:
- Temperature: Increasing the temperature can increase the rate of evaporation and melting, potentially shifting the equilibrium towards more gas or liquid, depending on the system.
- Pressure: In systems with gases, increasing pressure often shifts equilibrium towards the phase with fewer molecules, while decreasing pressure can favor the phase with more molecules.
Understanding equilibrium in multi-phase systems is vital in various fields, such as chemical reactions, environmental science, and even industrial processes, where controlling phase changes is essential for efficiency and safety.
Real-World Applications of Matter States
The behavior of substances in different forms plays a crucial role in everyday life and industrial processes. From the solid form of metals in construction to the gaseous state in weather patterns, the transitions between these phases influence everything from manufacturing to environmental conditions. Understanding how substances interact in their various states enables innovations in a wide range of fields, from energy production to medical applications.
Industrial Processes
In the manufacturing world, manipulating substances in various phases is key to optimizing production and ensuring safety. For example:
- Freezing and Melting in Food Industry: Freezing techniques are commonly used to preserve food, relying on the transition from liquid to solid to lock in nutrients and extend shelf life. On the other hand, melting processes are used to prepare materials for packaging and storage.
- Metal Casting: Metals are often melted to form desired shapes in casting processes. The transition from solid to liquid allows metals to be poured into molds, and once cooled, they solidify to create a strong, durable product.
Environmental and Atmospheric Phenomena
The atmosphere and weather systems are directly influenced by the behavior of substances in different phases. For instance:
- Cloud Formation: Clouds are formed when water vapor in the air condenses into liquid droplets or ice crystals. This phase change is essential for precipitation, which impacts everything from water supply to agriculture.
- Boiling and Evaporation: The water cycle is a vital part of Earth’s environment. As water evaporates into the atmosphere and later condenses into clouds, the heat from the sun drives these phase transitions, maintaining the global climate system.
Technological Advancements
Technological innovations have harnessed the different phases of substances for everything from energy production to medical applications. For example:
- Supercooling in Electronics: Some electronic devices utilize cooling systems that rely on phase changes to dissipate heat. Supercooled liquids are used to maintain the temperature of sensitive components.
- Plasma Technology: Plasma, an ionized gas, has been used in technologies like plasma televisions and cutting-edge medical treatments, demonstrating the diverse applications of gases in high-energy states.
From the kitchen to the laboratory, the transitions between different forms of matter are central to technological and scientific advancement, helping solve complex challenges across industries and daily life.
Common Misconceptions About States
Many people hold incorrect beliefs about how substances behave in different forms. These misunderstandings can arise from oversimplified explanations or the complexity of scientific concepts. Recognizing and addressing these misconceptions is important for a clearer understanding of how substances transition between forms and how they function in various conditions.
Misconceptions About Solids, Liquids, and Gases
- Solids are completely rigid: While solids may seem rigid, they are still made up of particles that vibrate in place. The level of rigidity varies depending on the material’s structure, and certain solids, like rubber, can deform under stress.
- Liquids do not have a fixed shape: While it’s true that liquids take the shape of their container, they have a definite volume. Unlike gases, which can expand to fill any space, liquids maintain a fixed volume under normal conditions.
- Gases always expand to fill the container: Gases do expand to fill their container, but this does not mean that the particles have no structure. Gas molecules are in constant motion, but they still interact with one another, which affects their behavior under varying temperatures and pressures.
Myths About Phase Transitions
- Melting is the same as dissolving: Many people confuse melting with dissolving. Melting is the process where a solid turns into a liquid due to heat, whereas dissolving involves a solute integrating into a solvent, creating a homogeneous mixture.
- Boiling only happens at 100°C: While 100°C is the boiling point of water at standard atmospheric pressure, the boiling point of a liquid varies with changes in pressure. At higher altitudes, for example, water boils at a lower temperature.
- Condensation is always associated with cooling: While condensation typically occurs when a gas cools down and turns into a liquid, it can also happen when the gas is compressed. Temperature is not the only factor in phase transitions.
Understanding these misconceptions helps in forming a more accurate view of how different forms behave and how phase transitions occur in the real world. With a deeper knowledge, one can better grasp the processes that govern everything from everyday phenomena to industrial applications.
Reviewing Chapter 10 Key Concepts
In this section, we will recap the essential ideas covered in the study of different forms and their behavior under various conditions. These concepts are fundamental for understanding the transformations and interactions of substances. By exploring the characteristics of different forms and how they respond to changes in temperature, pressure, and other external factors, we can gain a deeper insight into their properties and applications.
Understanding the Forms and Their Properties
- Solid Characteristics: Solids have tightly packed particles that vibrate but do not move freely. This structure gives them a fixed shape and volume.
- Liquid Behavior: Liquids have a definite volume but take the shape of their container. The particles are more loosely packed than in solids, allowing them to flow.
- Gas Properties: Gas particles are widely spaced and move freely. They have neither a fixed shape nor volume, expanding to fill any available space.
Phase Transitions and External Influences
- Temperature and Pressure: These factors play a crucial role in how a substance changes from one form to another. Heating a solid can cause it to melt, while increasing pressure on a gas can turn it into a liquid.
- Equilibrium in Multiple Forms: At certain conditions, substances can exist in more than one form at the same time, maintaining a balanced state between the different phases.
- Energy and Transitions: Phase changes often involve the absorption or release of energy. This energy change is vital for understanding the behavior of substances when they transition from one form to another.
By revisiting these key ideas, we can reinforce our understanding of how substances behave under varying conditions and apply this knowledge to real-world scenarios. The principles of form and transition are not only essential in academic studies but also in everyday applications, from industrial processes to natural phenomena.