
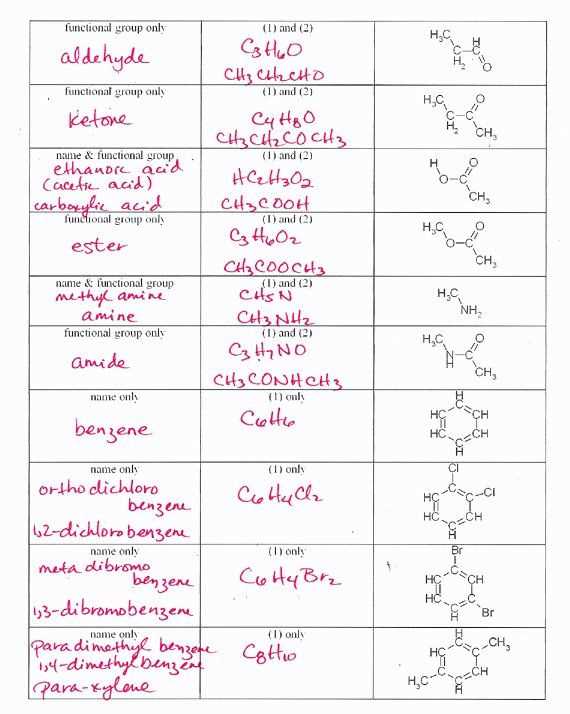
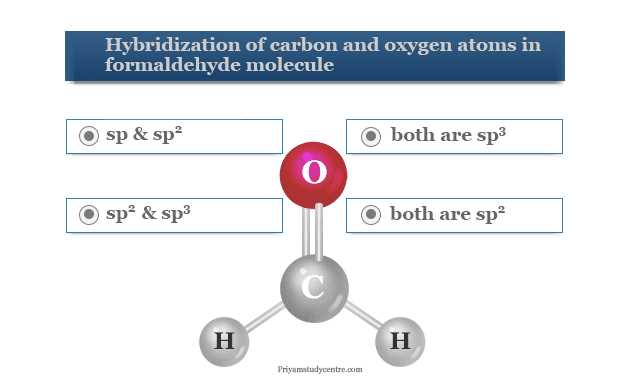
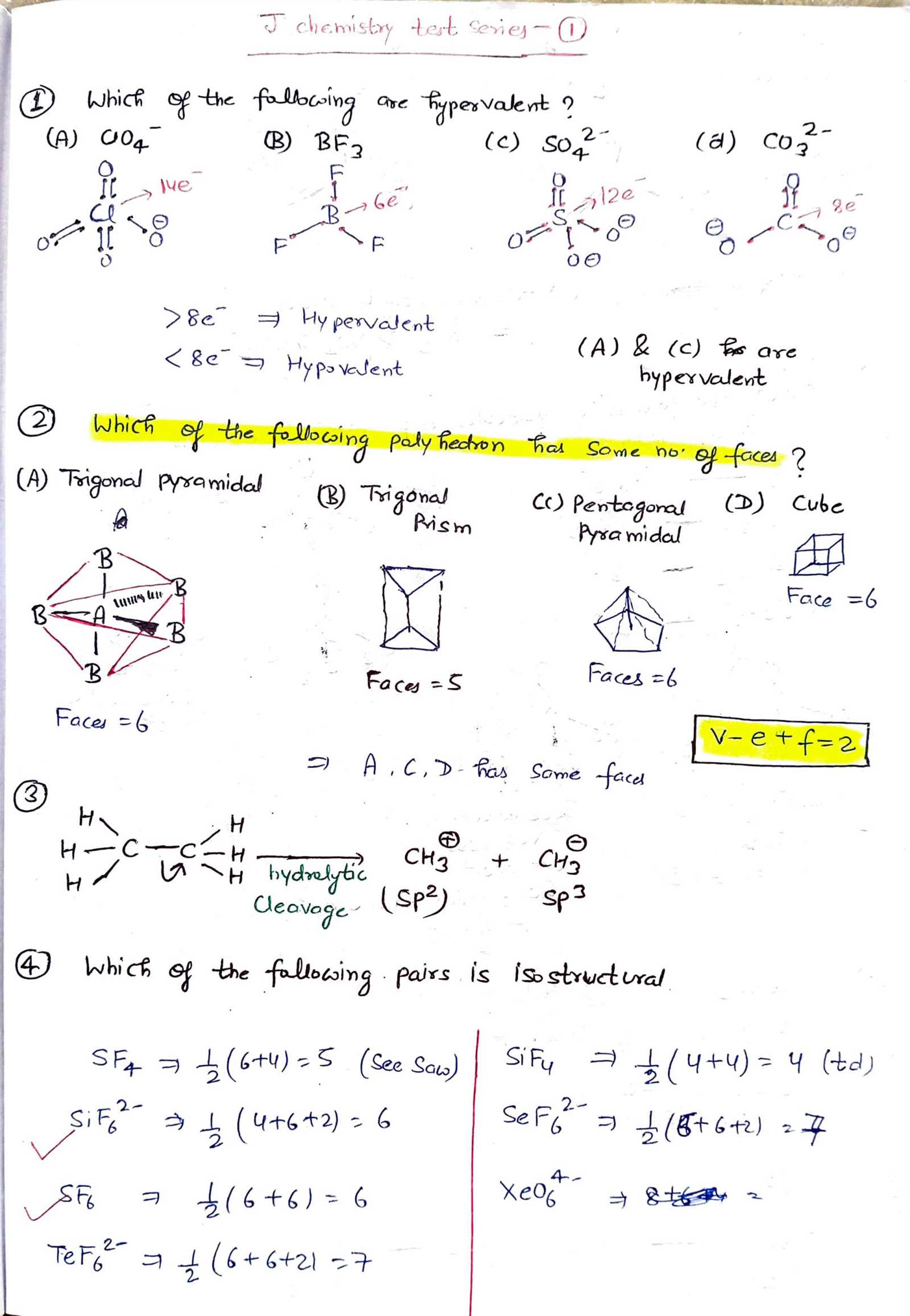
Understanding the nature of how atoms connect to form compounds is a crucial aspect of studying molecular science. It involves recognizing patterns that govern the formation and stability of various substances. A clear grasp of these concepts not only enhances theoretical knowledge but also prepares individuals for practical applications in the field.
In this section, we explore a variety of topics related to atomic connections, examining the principles behind their formation, the strength of their links, and how they influence the properties of materials. By breaking down these ideas, readers can approach complex concepts with clarity and confidence.
Throughout this guide, we will present a range of scenarios to practice key principles, ensuring a well-rounded understanding of molecular structure and interaction. Whether you’re new to the topic or revisiting it, this content aims to provide helpful insights for a comprehensive understanding of the subject.
Chemical Bonding Exam Questions and Answers
Preparing for assessments on molecular interactions requires a deep understanding of how atoms connect and form stable structures. The process of recognizing these interactions plays a central role in answering problems accurately. By studying the various types of atomic connections, individuals can approach challenges with greater clarity and confidence.
In this section, we will focus on providing valuable insight into common problems encountered during tests. We’ll explore how to identify different types of atomic links, how to predict their behaviors, and what factors influence their formation. The key is to approach each task systematically, applying theoretical knowledge to practical scenarios.
To strengthen your grasp on the subject, practice exercises are included, designed to reflect the most common scenarios presented in evaluations. These exercises will guide you in applying the fundamental principles to real-world examples, offering a chance to test your knowledge and improve problem-solving skills.
Understanding the Basics of Chemical Bonds
At the heart of molecular science lies the concept of how atoms come together to form stable units. The interactions between atoms determine the properties and behavior of matter. These connections are essential in shaping everything from simple molecules to complex compounds, affecting how substances react in different environments.
To grasp these interactions fully, it is important to explore the different types of connections that can form between atoms. Some are stronger, others weaker, but all play significant roles in determining the stability and reactivity of the material. Understanding these foundational concepts is crucial for analyzing various substances and predicting their behavior in different scenarios.
By learning the basic principles of atomic interactions, you will be able to build a solid foundation for more advanced topics. With a clear understanding of these concepts, solving related challenges becomes a more structured and logical process.
Types of Chemical Bonds Explained
The interactions between atoms are not all the same; they vary based on how atoms share or transfer electrons. These differences influence the properties of the resulting compounds. Some connections result in rigid, stable structures, while others allow for more flexibility and movement. Understanding the distinctions between these types is essential for analyzing how substances will behave under different conditions.
The primary categories of atomic connections are those where electrons are shared, transferred, or delocalized. Each type brings unique characteristics to the compounds formed, impacting factors such as strength, conductivity, and reactivity. By recognizing these key differences, it becomes easier to predict how a material will respond in various environments.
Through this section, we will explore each type of interaction in detail, focusing on their formation, strengths, and typical characteristics. This knowledge will help in answering related challenges more effectively and with a deeper understanding of molecular behavior.
Common Questions on Ionic Bonds
Understanding how atoms interact to form stable structures is crucial, especially when it comes to the type of connection where electrons are transferred between particles. This process results in the creation of oppositely charged ions that attract each other, leading to the formation of a solid structure. Many people have inquiries about this process, from the nature of the connections to the behavior of these compounds in different conditions.
How Are Ions Formed?
When atoms gain or lose electrons, they become charged, forming ions. Metals typically lose electrons to become positively charged, while nonmetals gain electrons to become negatively charged. These charged particles are then attracted to each other, forming a stable unit. Understanding this process is essential for recognizing how certain substances behave in different environments.
What Are the Key Properties of Ionic Compounds?
Ionic compounds have several distinct properties, such as high melting and boiling points due to the strong forces between ions. They are also good conductors of electricity when dissolved in water or melted, as the ions become free to move. These characteristics are important for understanding how ionic substances perform in practical applications, from industrial processes to everyday use.
Covalent Bonding and Key Concepts
When atoms share electrons to achieve a more stable configuration, they form a strong connection that plays a vital role in the structure of many substances. This type of interaction is common in both organic and inorganic molecules. Understanding the key principles behind these connections is essential for analyzing how molecules behave in various environments and chemical reactions.
The Role of Electron Sharing
In covalent interactions, atoms share one or more pairs of electrons to fill their outer electron shells. This sharing allows each atom to attain a stable electron configuration, similar to that of the nearest noble gas. The number of electron pairs shared between atoms can vary, leading to single, double, or even triple connections, each affecting the strength and stability of the resulting compound.
Properties of Molecules with Shared Electrons
Substances formed through electron sharing tend to have distinct properties compared to those formed through other types of atomic connections. These molecules often have lower melting and boiling points, are typically non-conductive in their solid state, and are usually insoluble in water. Understanding these characteristics is key to predicting the behavior of compounds in different physical states and under varying conditions.
Polar vs Non-Polar Covalent Bonds
When atoms share electrons in a molecule, the distribution of those electrons can vary depending on the electronegativity of the atoms involved. This variation creates different types of connections that have distinct properties. In some cases, the electrons are shared equally, while in others, they are drawn more strongly toward one atom. The result is the formation of either polar or non-polar structures, each influencing the molecule’s behavior in unique ways.
Understanding Polar Connections
In polar interactions, the electrons are not shared equally between atoms. One atom, typically the more electronegative, attracts the electrons more strongly, creating a partial negative charge on that atom and a partial positive charge on the other. This imbalance leads to a dipole, where the molecule has distinct positive and negative ends. These connections affect the molecule’s solubility, reactivity, and interaction with other polar substances.
Exploring Non-Polar Interactions
Non-polar connections, on the other hand, involve equal sharing of electrons between atoms of similar electronegativity. Because the electrons are distributed symmetrically, the molecule does not have any significant charge separation. As a result, non-polar molecules tend to be more stable and have lower reactivity in certain environments. These compounds are often insoluble in water but can dissolve in other non-polar solvents.
How to Identify Metallic Bonds
When atoms form a connection by sharing their outer electrons, some elements exhibit a unique behavior where these electrons are not tightly bound to any specific atom. Instead, they form a “sea” of delocalized electrons that move freely throughout the structure. This type of interaction is characteristic of metallic materials and gives rise to several important properties that help in identifying these types of connections.
To identify a metallic connection, it’s important to look for a few key characteristics that are typical of metals. These include electrical conductivity, malleability, and high melting points. The mobility of electrons allows metals to conduct electricity well, while the flexibility of electron sharing makes them malleable and ductile.
| Property | Explanation |
|---|---|
| Electrical Conductivity | The free electrons move easily, allowing the flow of electricity through the material. |
| Malleability | The arrangement of atoms and free electrons allows the material to be hammered into thin sheets without breaking. |
| High Melting Point | Strong metallic bonds hold the atoms together, requiring high energy to break them apart. |
Electron Configuration in Bonding

The arrangement of electrons around an atom plays a crucial role in determining how atoms interact with each other. By understanding electron configurations, we can predict how atoms will connect, whether they will share, transfer, or delocalize electrons. This knowledge is vital in explaining the stability and reactivity of different compounds formed by atomic interactions.
The Role of Valence Electrons
The electrons in the outermost shell, known as valence electrons, are the primary players in the formation of connections between atoms. These electrons determine the atom’s ability to form bonds with other atoms. The closer an atom’s electron configuration is to that of a noble gas, the more stable the resulting structure will be. Some key points to consider:
- Atoms with one or two valence electrons often lose them to form positive ions.
- Atoms with five to seven valence electrons tend to gain electrons to form negative ions.
- Atoms with a complete set of valence electrons (eight) are less likely to form bonds.
Electron Configuration and Bonding Types
The type of interaction formed depends largely on how the valence electrons are arranged. Here are the basic types of atomic connections:
- Ionic interactions: Formed when an atom donates electrons and another accepts them, resulting in oppositely charged ions.
- Covalent interactions: Occur when atoms share electrons to achieve a full outer shell.
- Metallic interactions: Involve a ‘sea’ of delocalized electrons that move freely throughout the structure.
By understanding how electron configurations influence the behavior of atoms, we can better predict how they will interact and the properties of the compounds they form.
Bond Length and Bond Energy
The distance between atoms in a molecule and the strength of the connection between them are key factors in determining the stability and reactivity of compounds. These two aspects, length and energy, are intimately related to the way atoms share or transfer electrons, influencing the properties of the material. Understanding how these two factors work together helps to explain how substances behave under different conditions.
Understanding Bond Length
Bond length refers to the average distance between the nuclei of two connected atoms. This distance is influenced by the size of the atoms involved and the type of connection formed. Typically, smaller atoms form shorter connections, while larger atoms form longer connections. The strength of the interaction also plays a role–stronger interactions tend to result in shorter bonds. This relationship is important when considering how molecules behave during reactions or in different environments.
Exploring Bond Energy
Bond energy is the amount of energy required to break a connection between two atoms. It is a measure of the strength of the bond–the higher the energy needed to break the bond, the stronger the connection. The energy required is influenced by factors such as the number of shared electrons and the size of the atoms involved. Generally, bonds formed by smaller atoms or with multiple electron pairs tend to have higher bond energies. This concept is vital for understanding how substances react with one another and how stable they are in various conditions.
Resonance Structures and Bonding
In some molecules, the arrangement of electrons cannot be fully explained by a single structure. Instead, multiple possible configurations contribute to the overall structure, with each one representing a different way the electrons can be distributed. These different forms, known as resonance structures, help to describe the actual electron distribution in a molecule more accurately. This concept plays a key role in understanding the stability and reactivity of certain compounds.
Understanding Resonance
Resonance occurs when a molecule can be represented by two or more valid structures that differ only in the placement of electrons. Instead of existing as one fixed form, the molecule is considered to be a hybrid of these structures, where the true electron arrangement is an average of all possible configurations. Some important points about resonance:
- Resonance structures are not isomers; they simply represent different ways of distributing electrons within the same molecule.
- The actual structure is more stable than any individual resonance form.
- Resonance helps explain the stability of molecules that cannot be accurately described by a single structure.
Impact on Molecular Properties

The presence of resonance can influence several important properties of a molecule. For example, it affects the bond length, as the electron density is spread out more evenly, often leading to bonds that are stronger and shorter than predicted by a single structure. Additionally, resonance helps explain the delocalization of electrons, contributing to the molecule’s stability and its ability to interact with other molecules. This phenomenon is especially important in aromatic compounds and in molecules with conjugated systems.
Lewis Structures and Bonding Patterns
The arrangement of atoms in a molecule and the distribution of electrons are essential to understanding the molecule’s structure and behavior. One way to visually represent these aspects is through Lewis structures, which provide a clear depiction of how atoms are connected and where the electrons are shared or paired. These structures allow us to predict molecular geometry, reactivity, and other properties based on how electrons are arranged within the system.
Constructing Lewis Structures
To draw a Lewis structure, the first step is to determine the total number of valence electrons available from all the atoms involved. These electrons are then used to form bonds and lone pairs. Each single bond represents two shared electrons. When constructing these structures, it is important to follow certain rules to ensure accuracy:
- Count all valence electrons for the atoms in the molecule.
- Place electrons in pairs, with each atom aiming to achieve a full outer shell, often referred to as the octet rule.
- Use double or triple bonds if necessary to fulfill the octet for atoms with fewer electrons.
- Place any remaining electrons as lone pairs on atoms that need them.
Bonding Patterns in Molecules
Lewis structures reveal not just the arrangement of atoms but also the type of interactions between them. Different types of bonds–single, double, or triple–can form between atoms, depending on how many electron pairs are shared. Additionally, some atoms may have lone pairs of electrons that affect the overall shape and polarity of the molecule. Recognizing these patterns helps in understanding the molecular geometry and reactivity of the compound.
How Electronegativity Affects Bonding
The way atoms interact with each other in a molecule depends heavily on their ability to attract electrons. This tendency varies across different elements and plays a crucial role in determining the nature of the connection formed between atoms. When two atoms form a link, the difference in their ability to attract shared electrons can influence the bond’s characteristics, such as polarity and strength. Understanding this property helps explain many molecular behaviors and properties.
Electronegativity is a measure of an atom’s ability to pull electrons towards itself when it is part of a compound. The greater the difference in electronegativity between two atoms, the more likely the electrons will be unevenly distributed. If one atom is significantly more electronegative than the other, it will attract the shared electrons more strongly, leading to a polar connection. In cases where the electronegativity difference is very small, the electrons are more evenly shared, resulting in a nonpolar connection.
Overall, the electronegativity of atoms plays a key role in shaping the physical and chemical properties of molecules. It affects not only the nature of the bond itself but also factors such as molecular polarity, reactivity, and solubility. Recognizing the impact of electronegativity is essential for predicting how molecules will behave in different conditions and environments.
Practical Examples of Chemical Bonds
The formation of connections between atoms plays a fundamental role in determining the properties of various substances. These connections, or links, are not only critical in understanding basic molecular structures but also essential in practical applications that affect everyday life. By observing how atoms join together, we can better grasp how materials behave under different conditions, whether in nature or in engineered environments.
Examples in Everyday Life
From the water we drink to the metals used in construction, atomic connections are at the heart of countless materials. Here are a few practical examples:
- Water (H₂O): The interaction between hydrogen and oxygen atoms forms a polar connection, giving water its unique properties like high surface tension and its solvent capabilities.
- Salt (NaCl): Sodium and chlorine atoms form an ionic link, which gives table salt its crystalline structure and ability to dissolve in water, contributing to its use in food and industrial applications.
- Diamond (C): Carbon atoms in a diamond are linked by strong covalent bonds, resulting in a material with exceptional hardness and thermal conductivity, used in jewelry and cutting tools.
Industrial and Technological Uses
Understanding how atoms connect also plays a crucial role in technology and industry. Examples include:
- Steel Manufacturing: The combination of iron atoms with carbon through metallic bonds creates steel, a material known for its strength and versatility in construction and manufacturing.
- Battery Technology: In lithium-ion batteries, lithium atoms bond with oxygen, allowing for energy storage and release, which powers devices like smartphones and electric vehicles.
- Semiconductors: Silicon atoms, connected by covalent bonds, form the foundation of most electronic devices, including computers, smartphones, and solar panels.
Common Mistakes in Bonding Questions
When tackling topics related to atomic connections, many individuals often encounter misunderstandings that can lead to incorrect conclusions. These errors typically arise due to a lack of clarity about the underlying principles or an oversimplification of complex concepts. Recognizing these common mistakes is crucial for anyone aiming to master the subject, as it helps to avoid confusion and improves accuracy in problem-solving.
Typical Errors to Watch Out For
Below are some frequent mistakes that students or learners make when dealing with atomic interactions:
- Confusing Ionic and Covalent Bonds: One of the most common errors is mixing up ionic and covalent links. Ionic connections occur when electrons are transferred, while covalent bonds involve the sharing of electrons. Misunderstanding this distinction can lead to incorrect predictions about the properties of substances.
- Incorrect Electron Count: Failing to account for the proper number of electrons in a molecule or ion is a typical mistake. This often occurs when drawing diagrams or when trying to determine the molecular geometry, which can lead to incorrect representations.
- Overlooking Electronegativity: Not considering the electronegativity difference between atoms can cause errors in predicting bond type. A large difference typically results in an ionic bond, whereas a small difference leads to a covalent bond. Ignoring this factor can mislead conclusions.
- Misinterpreting Resonance Structures: Resonance is often misunderstood. Failing to recognize when multiple structures are needed to describe a molecule can result in an incomplete or inaccurate representation.
- Assuming Bonds Are Always Fixed: A common misconception is assuming that atomic connections are always static and fixed in length. In reality, bonds can fluctuate, especially in larger molecules or under different conditions.
How to Avoid These Mistakes
To prevent these common errors, it’s essential to focus on understanding the core principles of atomic interactions and how different factors influence the way atoms combine. Practice and careful analysis of each problem will help in reducing misunderstandings and improving accuracy in identifying and explaining these connections.
Tips for Answering Bonding Exam Questions
When preparing for assessments on atomic interactions, the key to success lies in clear understanding and systematic approaches. Efficiently addressing problems that focus on how atoms form connections can sometimes be tricky. However, by adopting the right strategies, it becomes easier to identify patterns, solve problems, and showcase knowledge effectively. Below are some useful tips to enhance your performance when tackling such topics.
Steps to Enhance Your Response
Use the following methods to approach each task systematically:
- Read the Question Carefully: Ensure you fully understand what is being asked before jumping into your response. Pay attention to terms like “identify”, “explain”, or “describe”, as they indicate the level of detail needed.
- Break Down the Problem: For complex scenarios, break the question into smaller parts. Address each part individually before integrating your findings into a complete answer.
- Use Diagrams Where Appropriate: Visual aids such as electron diagrams, molecular structures, or bond formations can clarify your explanation. They also provide evidence of your understanding, which may help you score additional marks.
- Show Your Work: In problems involving calculations or reasoning, ensure you demonstrate the steps taken to reach your solution. This shows the examiner how you arrived at your answer and allows partial credit if a mistake was made.
- Stay Focused on Key Concepts: Identify and highlight the main principles related to the topic. Whether it’s the types of atomic connections or specific properties like polarity, clarity and precision are critical.
Time Management Tips
Effective time management can make a significant difference in your overall performance. Keep these guidelines in mind during your test:
| Tip | Explanation |
|---|---|
| Allocate Time for Each Question | Estimate how much time each section should take and stick to it. Don’t get bogged down in one difficult question. |
| Review Your Answers | Reserve the last few minutes to check your work. Correcting small errors can often lead to higher marks. |
| Prioritize Simpler Questions | If there are multiple questions, start with the ones you find easiest. This builds confidence and ensures you secure marks early on. |
By following these tips, you will be better equipped to address each task methodically and demonstrate a solid grasp of the subject. Practice regularly to refine these skills and boost your performance in assessments.
Analyzing Molecular Shapes and Bonds
Understanding the arrangement of atoms in a molecule is essential for predicting its properties and behavior. The spatial orientation of atoms and the nature of their connections are key factors that determine how a substance interacts with others. By analyzing these configurations, we can gain insight into the molecule’s reactivity, polarity, and physical characteristics.
The arrangement of atoms within a molecule is not random but follows specific patterns based on the interactions between atoms and their electrons. These patterns, often described using geometric models, play a crucial role in explaining why molecules have particular shapes, such as linear, bent, or tetrahedral. Analyzing these molecular geometries is crucial when determining how molecules interact with each other in various chemical processes.
By studying the strength and nature of atomic interactions, one can predict the properties of substances and how they behave under different conditions. For example, the difference in electronegativity between two atoms will affect whether they share electrons equally or unequally, leading to different types of connections that influence the overall molecular structure.
Practice Questions for Bonding Exams
One of the best ways to prepare for assessments on atomic interactions is by practicing problems that challenge your understanding of how atoms connect and interact with one another. These exercises help reinforce key concepts and improve your problem-solving skills by testing your ability to apply theoretical knowledge to real-world scenarios. By reviewing such practice problems, you can identify areas where you need more focus and refine your approach to different types of connections between atoms.
Example Problem 1
Consider two elements, one with high electronegativity and one with low electronegativity. Describe the nature of the interaction between these two elements and explain how it affects the properties of the resulting compound.
Example Problem 2
Given a molecule with a trigonal planar shape, predict its geometry based on the number of electron pairs around the central atom. How does this arrangement influence the molecule’s reactivity?
These problems test a variety of core ideas related to molecular structure, including the types of atomic interactions, electron distribution, and how these factors impact the overall behavior of molecules. Practicing problems like these will help you prepare for assessments and strengthen your grasp of complex topics related to atomic relationships.