
As the time approaches for your next evaluation, it’s essential to revisit key concepts and refine your understanding of the material. This section will help you focus on the most important areas and ensure you’re well-prepared for the challenges ahead. Whether you’re looking to brush up on complex theories or strengthen your problem-solving skills, these strategies will support your progress.
Comprehending core principles is crucial, as many of the questions will test your ability to apply knowledge in real-world contexts. It’s important to go beyond memorization and understand the underlying processes that govern various topics. Equally vital is practicing problem-solving techniques that will help you work through different scenarios efficiently.
In the following sections, you will find valuable insights on topics that are commonly tested. With consistent review and thoughtful application of these strategies, you’ll gain the confidence needed to approach the assessment with assurance and clarity.
Preparation for Your Upcoming Test
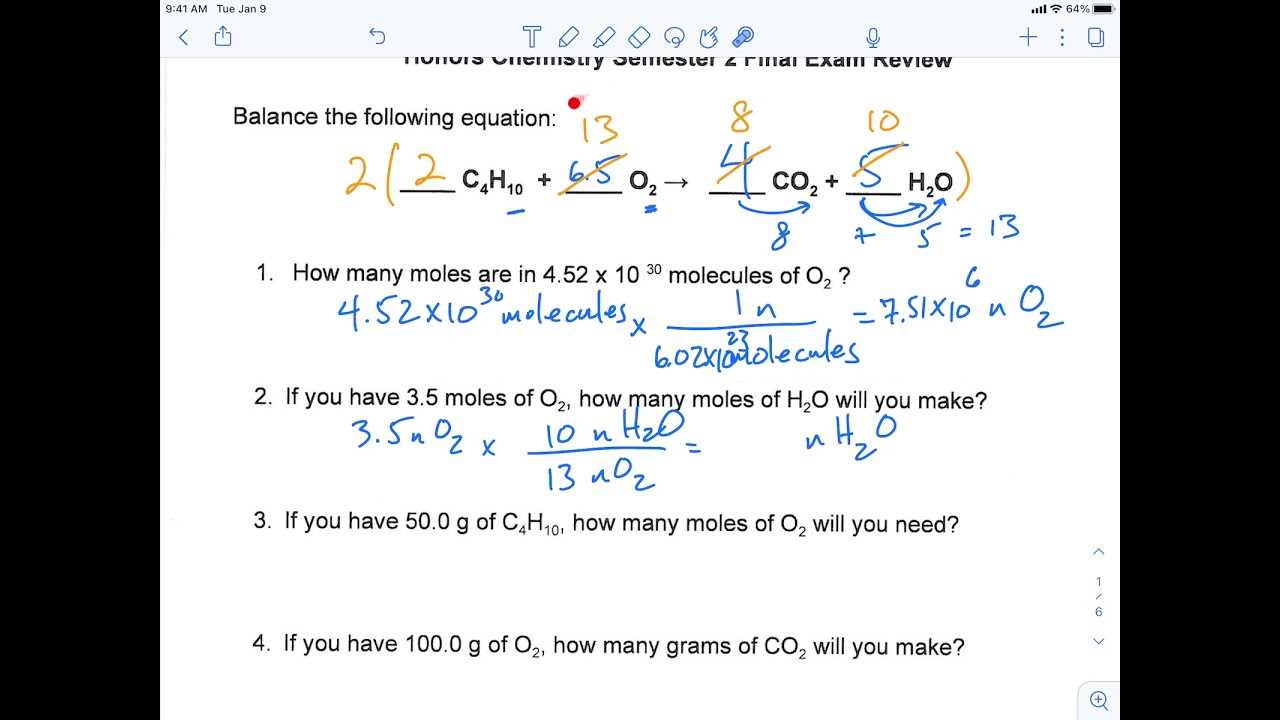
Success in any evaluation requires a clear understanding of essential topics and the ability to apply that knowledge effectively. By focusing on the most relevant concepts and honing your skills in practical problem-solving, you’ll be better equipped to tackle the challenges ahead. This section will help you prioritize the areas that are likely to make the greatest impact on your performance.
Mastering fundamental ideas is the first step in preparing for any rigorous assessment. It’s important to recognize the key themes that consistently appear and understand how they connect to one another. Additionally, practicing with sample problems will give you the confidence to apply your knowledge under pressure and improve your accuracy in answering questions.
As you work through the material, focus on improving your ability to analyze complex problems and draw conclusions based on your understanding. Consistent review and thoughtful application of these concepts will help you build a strong foundation and perform at your best when the time comes.
Key Topics to Focus On
When preparing for any important assessment, it’s essential to concentrate on the most relevant and commonly tested concepts. By identifying the core areas of study, you can focus your efforts on understanding the fundamental principles and how they apply in various scenarios. The following topics are key to building a strong foundation and performing at your best.
Core Principles to Master
It’s critical to have a firm grasp of the fundamental concepts that serve as the building blocks for more complex ideas. These topics form the basis for many of the problems you’ll encounter and understanding them will allow you to approach questions with confidence.
Commonly Tested Areas
These areas often appear in assessments and are integral to demonstrating your comprehensive understanding. Mastering these will ensure you’re well-prepared for various question formats, from theoretical to practical applications.
| Topic | Description |
|---|---|
| Atomic Structure | Understanding the arrangement and behavior of atoms is essential for explaining reactions and properties of materials. |
| Reactions and Equations | Familiarity with how different substances interact and how to balance equations is key for many questions. |
| Stoichiometry | Ability to perform calculations involving reactants and products is crucial for solving quantitative problems. |
| Thermodynamics | Grasping the laws of energy and heat transfer helps explain the behavior of substances under different conditions. |
Understanding Chemical Reactions
Grasping how substances interact and transform into new materials is a cornerstone of scientific inquiry. In any assessment, a solid understanding of reaction mechanisms is essential for explaining the processes that drive these transformations. Mastering this area will help you confidently approach questions on the behavior of different compounds and their resulting products.
Types of Reactions
Recognizing the different types of reactions is crucial for understanding the specific changes that occur during each process. From synthesis to decomposition, each reaction follows its own distinct pathway, governed by particular rules and principles.
Balancing Equations
One of the most critical skills in working with reactions is the ability to balance equations. This process ensures that the law of conservation of mass is upheld, and it provides insight into how substances interact in a given reaction. Being proficient in balancing will make problem-solving more straightforward and help you better understand the quantitative aspects of reactions.
Essential Periodic Table Concepts
Understanding the organization of elements is fundamental to grasping the behavior and properties of substances. The periodic table is a powerful tool that allows us to predict trends, identify relationships between elements, and comprehend how they interact in various processes. A solid grasp of its key concepts is essential for making sense of the material around us.
Element Classification plays a critical role in determining an element’s characteristics and its reactivity. Elements are grouped based on their similar properties, and recognizing these groups helps in predicting behavior during reactions. From metals to nonmetals, each category has distinct features that define how they react and combine with other elements.
Trends Across the Table are another crucial aspect. As you move across periods or down groups, the properties of elements change in predictable ways. Understanding these trends–such as electronegativity, atomic radius, and ionization energy–helps explain the differences in reactivity and bonding among elements.
Molecular Structure and Bonding Basics

Understanding the arrangement of atoms within a molecule and how they interact through bonds is key to predicting the properties and behavior of different substances. The way atoms are held together determines their stability, reactivity, and the overall structure of the material. A solid grasp of these concepts is essential for analyzing chemical reactions and explaining the behavior of compounds.
There are several types of bonds that form between atoms, each with distinct characteristics. These interactions influence everything from the physical state of a substance to its chemical reactivity. The following are key bonding types:
- Covalent Bonds: Atoms share electrons to form molecules, typically between nonmetals.
- Ionics Bonds: One atom donates an electron to another, creating charged particles that attract each other.
- Metallic Bonds: Electrons move freely between metal atoms, resulting in a ‘sea of electrons’ that provide electrical conductivity.
In addition to bonding, the arrangement of atoms in three-dimensional space is crucial. This molecular structure influences the physical and chemical properties of substances, such as boiling and melting points, solubility, and reactivity. Understanding how atoms are positioned in a molecule helps predict how a compound will interact in different environments.
Mastering Stoichiometry Calculations
Being able to accurately perform calculations related to the quantities of substances involved in chemical reactions is a vital skill. These calculations allow us to predict how much of each reactant is required and how much product will be formed. Mastering this area not only aids in solving problems but also deepens understanding of how different substances interact during reactions.
At its core, stoichiometry involves using balanced chemical equations to convert between different units, such as moles, mass, and volume. This process requires careful attention to detail and the ability to apply conversion factors accurately. By practicing these calculations, you develop a clearer picture of how atoms are conserved throughout a reaction and how the quantities of reactants and products relate to one another.
Whether you’re dealing with simple or complex reactions, consistently following a step-by-step approach to stoichiometric calculations will help ensure accuracy and boost your confidence when tackling related problems.
Acids and Bases Explained

Understanding the behavior of acids and bases is crucial for explaining many chemical reactions and processes. These two classes of compounds exhibit distinct properties that determine how they interact with other substances. Whether in neutralization reactions or in various industrial and biological applications, knowing how acids and bases function is essential for a comprehensive understanding of many scientific concepts.
Acids and bases are often categorized based on their ability to donate or accept specific particles in reactions. Here are some key characteristics:
- Acids: Typically, acids release hydrogen ions (H+) when dissolved in water, making the solution acidic. Common traits include a sour taste and the ability to react with metals.
- Bases: Bases, on the other hand, accept hydrogen ions or release hydroxide ions (OH–). They usually have a bitter taste and slippery texture, often found in substances like soaps and cleaning agents.
One important aspect of these compounds is their ability to neutralize each other. When an acid reacts with a base, the result is typically water and a salt, which can be critical in maintaining balance in biological systems or in laboratory processes.
Understanding how acids and bases influence the pH of solutions is also fundamental. The pH scale, which ranges from 0 to 14, helps measure the acidity or alkalinity of a solution. A pH of 7 is neutral, while values below 7 indicate acidic conditions, and values above 7 represent basic conditions.
Important Thermodynamics Principles
Understanding the fundamental principles of energy transfer and transformation is essential for explaining many processes in both natural and artificial systems. These principles describe how energy flows and how it can be harnessed or converted, which is crucial for everything from industrial applications to biological processes. By mastering these concepts, you gain a deeper understanding of the world around you and how various systems maintain balance.
Key concepts in thermodynamics include the conservation of energy, the direction of energy flow, and the relationship between heat and work. These ideas provide a framework for analyzing how systems evolve over time, including predicting whether reactions will occur spontaneously or if energy input is required. Below are some of the core principles to focus on:
- First Law of Thermodynamics: Energy cannot be created or destroyed, only transformed from one form to another. This law is essential for understanding energy conservation in any system.
- Second Law of Thermodynamics: The total entropy (disorder) of an isolated system will always increase over time, which explains the natural tendency of systems to move towards equilibrium.
- Third Law of Thermodynamics: As the temperature of a system approaches absolute zero, the entropy of the system approaches a minimum value, often reaching zero in perfect crystals.
These principles form the foundation for a wide range of scientific and practical applications. From designing engines to understanding climate change, thermodynamics helps explain how energy behaves in a variety of systems.
Understanding Chemical Kinetics
Grasping how reactions occur over time and what factors influence the rate of transformation is essential for understanding many processes in both natural and engineered systems. The speed at which reactants turn into products depends on a variety of factors, including temperature, concentration, and the presence of catalysts. By mastering these concepts, you can better predict how a reaction will unfold under different conditions.
Several key elements determine the rate at which reactions proceed. These include the following:
- Concentration of Reactants: Higher concentrations of reactants typically lead to faster reactions, as there are more particles available to collide and interact.
- Temperature: Increasing temperature generally increases the rate of a reaction by providing more energy to the particles, causing them to collide more often and with greater energy.
- Catalysts: Catalysts speed up reactions without being consumed in the process. They lower the activation energy required for the reaction to occur.
- Surface Area: A larger surface area of reactants allows for more collisions between particles, which can increase the rate of reaction.
Understanding the relationship between these factors and the rate of a reaction is crucial for controlling chemical processes. By manipulating these variables, it is possible to optimize reactions for desired outcomes, whether in industrial settings or natural systems.
Organic Chemistry Fundamentals
The study of carbon-based compounds is a central branch of science that plays a crucial role in understanding a wide range of biological, environmental, and industrial processes. Carbon’s unique ability to form stable bonds with other elements allows for the creation of diverse molecules, from simple gases to complex biomolecules. This field explores how these compounds interact, their structures, and how they can be synthesized or transformed.
Carbon Bonding and Molecular Structures
One of the fundamental concepts in this field is the bonding properties of carbon atoms. Carbon atoms can form single, double, and triple bonds with other atoms, allowing for a variety of molecular structures. These structures can be chains, rings, or networks, each influencing the physical and chemical properties of the compounds. The most common types of organic molecules include:
- Hydrocarbons: Compounds made up of only carbon and hydrogen atoms. They can be classified as alkanes, alkenes, or alkynes based on the types of bonds between carbon atoms.
- Functional Groups: Specific groups of atoms that give molecules characteristic properties and reactivity. Examples include hydroxyl, carbonyl, and amino groups.
Reactivity and Functional Group Chemistry

Organic compounds exhibit a wide range of reactivity based on the functional groups they contain. The behavior of these compounds in various reactions, such as substitution, addition, or elimination reactions, is determined by the structure and properties of their functional groups. Understanding these reactions allows for the synthesis of new molecules with specific desired characteristics.
Electrochemistry Overview
Electrochemistry is a branch of science that explores the relationship between electricity and chemical reactions. It focuses on how electrical energy can be generated from chemical reactions, or conversely, how electrical energy can drive chemical changes. This field plays a key role in many practical applications, such as batteries, fuel cells, corrosion, and electroplating.
Redox Reactions and Electron Transfer
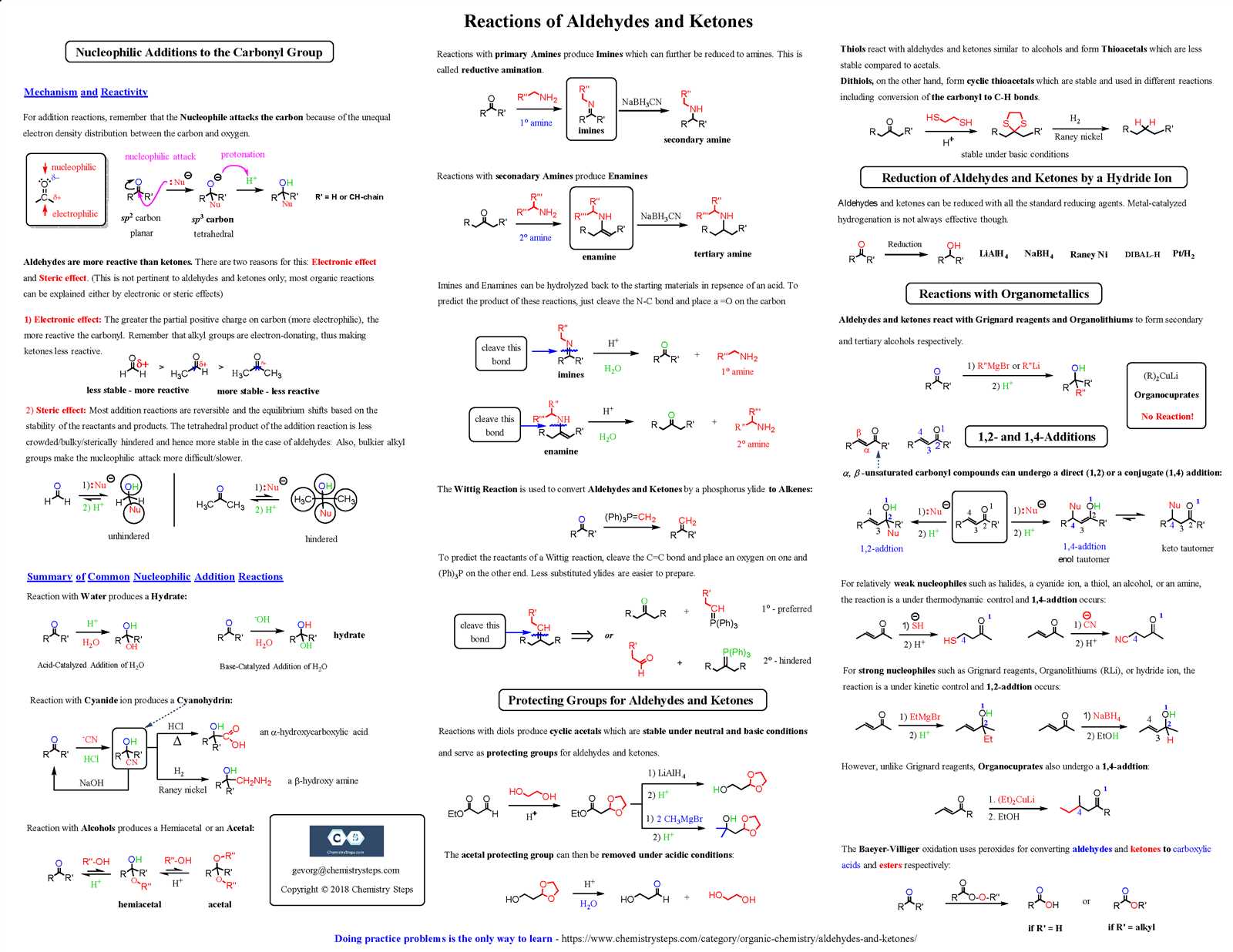
At the heart of electrochemistry lies the concept of redox reactions, where one substance gains electrons (reduction) and another loses electrons (oxidation). These reactions are fundamental in processes such as energy production and metal corrosion. Understanding how electrons move between substances allows us to predict how electrical current can be generated or how reactions can be controlled.
- Oxidation: The process where a substance loses electrons, often resulting in an increase in oxidation state.
- Reduction: The gain of electrons by a substance, leading to a decrease in oxidation state.
Applications in Technology and Industry
Electrochemical processes have a wide range of applications. For example, in batteries, chemical reactions generate electricity, while in electroplating, electrical energy is used to deposit metal onto surfaces. Fuel cells, which are becoming increasingly important as a source of clean energy, convert chemical energy directly into electrical energy through electrochemical reactions.
Critical Lab Techniques to Know
Mastering laboratory techniques is essential for conducting experiments accurately and safely. The ability to handle instruments, measure substances, and analyze results correctly ensures reliable outcomes in various scientific investigations. Whether in academic research, industry, or practical applications, proficiency in key lab methods is critical for success.
Key Techniques for Accurate Measurements
In the lab, precise measurements and proper handling of equipment are vital to obtain valid data. Common techniques include titration, filtration, and spectroscopy, each requiring a strong understanding of procedure and equipment use. Below is an overview of some essential methods:
| Technique | Description |
|---|---|
| Titration | A method used to determine the concentration of a substance in solution by adding a known volume of titrant until the reaction reaches its endpoint. |
| Filtration | Used to separate solid particles from a liquid or gas by passing the mixture through a filter medium. |
| Spectroscopy | A technique that measures the interaction of light with matter to identify the composition of substances or determine concentration. |
Safety and Precision in the Laboratory
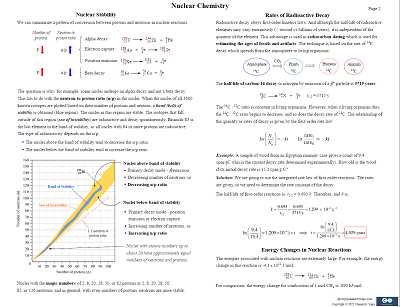
In addition to technical skills, lab safety and the ability to follow protocols are crucial. Proper use of protective equipment, understanding chemical hazards, and maintaining a clean workspace all contribute to a successful experiment. By mastering both the technical and safety aspects, lab work becomes more efficient and effective.
Balancing Chemical Equations
Balancing chemical reactions is a critical skill that ensures the law of conservation of mass is upheld. Every reaction involves a transformation of reactants into products, and the number of atoms of each element must remain constant before and after the reaction. Achieving this balance requires adjusting the coefficients of the substances involved, ensuring that each element’s quantity is the same on both sides of the equation.
The process of balancing begins by identifying the unbalanced equation, then adjusting the coefficients step-by-step. Typically, it’s best to start with the elements that appear in only one reactant and one product, then proceed to more complex parts of the equation. Finally, ensure that the smallest possible whole number coefficients are used.
Here is an example of balancing a basic reaction:
Unbalanced Equation: H2 + O2 → H2O
Balanced Equation: 2H2 + O2 → 2H2O
In this example, the hydrogen atoms and oxygen atoms are balanced on both sides of the equation by adjusting the coefficients. Through practice, balancing becomes more intuitive, and the process becomes an essential part of understanding and performing reactions in any scientific context.
Solubility and Solution Chemistry
The process of dissolving substances to form homogeneous mixtures is fundamental to many chemical processes. Understanding the principles that govern the formation of solutions and how different factors affect solubility is essential for predicting the behavior of substances in various environments. Solubility refers to the ability of a substance to dissolve in a solvent, and this phenomenon is influenced by factors such as temperature, pressure, and the nature of the solute and solvent.
When a solute dissolves in a solvent, it separates into individual molecules or ions, creating a solution. The amount of solute that can dissolve in a given amount of solvent depends on both the chemical properties of the solute and the solvent, as well as external conditions such as temperature and pressure. For example, gases tend to be more soluble in liquids at lower temperatures and higher pressures.
Below is a table summarizing some key factors that influence solubility:
| Factor | Effect on Solubility |
|---|---|
| Temperature | In general, the solubility of most solids increases with temperature, while the solubility of gases decreases. |
| Pressure | Increasing pressure generally increases the solubility of gases in liquids, but has little effect on the solubility of solids and liquids. |
| Nature of Solute and Solvent | Substances with similar chemical properties (e.g., polar solutes and solvents) tend to dissolve better in each other. |
Understanding these factors allows for better control of solution concentrations and is crucial in various applications, from pharmaceuticals to industrial processes. The behavior of solutes and solvents in different conditions is key to optimizing reactions and achieving desired outcomes in both laboratory and real-world settings.
Equilibrium and Le Chatelier’s Principle
In reversible reactions, a state of balance is achieved when the rates of the forward and reverse reactions are equal. This dynamic equilibrium is a critical concept in understanding how reactions reach stability and how changes in conditions affect the system. The idea that a system will adjust to counteract any changes imposed upon it is central to predicting how reactions will behave under different conditions.
Le Chatelier’s Principle helps explain how a system at equilibrium responds to changes in temperature, pressure, or concentration of reactants or products. When a disturbance occurs, the system shifts to restore balance by favoring either the forward or reverse reaction, depending on the nature of the change. This principle allows us to predict how a reaction will shift under various circumstances, making it crucial in both laboratory and industrial settings.
Key factors influencing equilibrium and how systems adjust include:
- Concentration Changes: If the concentration of reactants or products is altered, the system will shift to oppose the change by favoring the reaction that consumes or produces the affected substance.
- Temperature Changes: An increase in temperature typically favors the endothermic direction (absorbing heat), while a decrease favors the exothermic direction (releasing heat).
- Pressure Changes: For reactions involving gases, increasing pressure will shift the equilib
Practice with Sample Questions
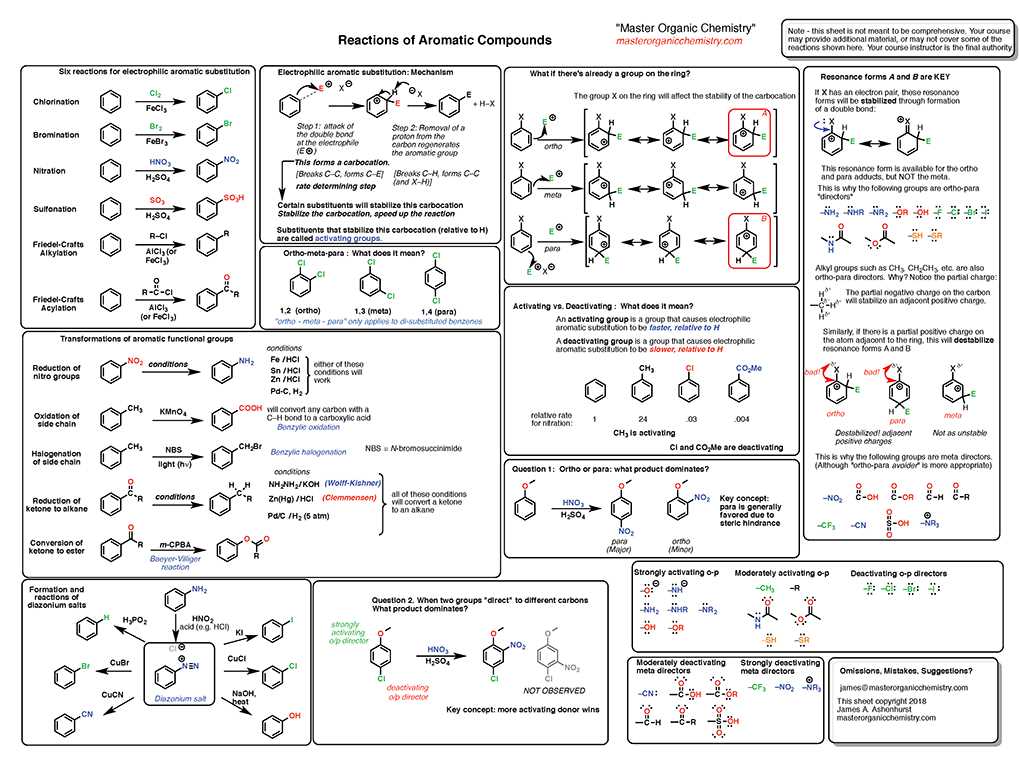
Reinforcing your understanding through practical exercises is a valuable way to solidify key concepts. Working through sample problems helps you apply theoretical knowledge to real-world scenarios, improving both your problem-solving skills and confidence. Practicing with a variety of questions can also highlight areas that need further focus and refinement.
Sample Question 1: Reaction Balancing
Balance the following chemical reaction:
Fe + O2 → Fe2O3
To solve this, start by balancing the elements on both sides of the equation. For example, you need to ensure the number of iron (Fe) atoms on the left matches those on the right side. Then, adjust the oxygen molecules and finally check all elements again to ensure the equation is balanced.
Sample Question 2: Mole Concept
How many grams of oxygen are required to react with 10 grams of hydrogen to form water?
This question involves applying the mole concept to determine how much of one substance is needed to completely react with another. First, calculate the number of moles for hydrogen, then use the stoichiometry of the balanced reaction to find out how many moles of oxygen are required. Finally, convert moles of oxygen into grams using its molar mass.
By working through these examples, you can enhance your problem-solving techniques and become more proficient in applying important principles. Repetition and variety in practice problems will help you improve your approach and prepare you for complex scenarios in the future.
Reviewing Key Formulas
Mastering important equations is crucial for solving complex problems efficiently. Knowing the right formulas and how to apply them is the foundation for tackling a wide range of questions. These formulas act as tools that allow you to quantify relationships between different variables and arrive at precise answers.
Below are some of the fundamental formulas that will help you navigate through various challenges:
- Molarity Formula: M = n / V
Where M is molarity, n is the number of moles of solute, and V is the volume of solution in liters.
- Ideal Gas Law: PV = nRT
This equation relates the pressure (P), volume (V), and temperature (T) of a gas with the number of moles (n) and the universal gas constant (R).
- Percentage Yield: % Yield = (Actual Yield / Theoretical Yield) × 100
Used to calculate the efficiency of a reaction, this formula compares the actual amount of product obtained to the theoretical maximum possible amount.
- Density Formula: ρ = m / V
Where ρ is density, m is mass, and V is volume.
- Heat Transfer Equation: q = mcΔT
Used for calculating the amount of heat energy (q) required to change the temperature of a substance, where m is mass, c is specific heat capacity, and ΔT is the change in temperature.
By regularly reviewing these key formulas and practicing their application, you’ll build a solid foundation to approach and solve problems with confidence. Understanding when and how to use each formula will make complex calculations much easier and faster to perform.
Study Tips for Success

Effective preparation requires more than just reading over materials. It’s about developing strategies that help you retain information and apply concepts with confidence. The following tips focus on creating efficient habits and techniques that can maximize your understanding and performance when facing complex topics.
Time Management and Organization
One of the most crucial elements for success is managing your time wisely. Organizing your sessions can help you stay on track and avoid last-minute cramming.
- Create a Schedule: Break down the material into manageable sections and allocate specific times for each. Stick to the plan to ensure consistent progress.
- Prioritize Key Concepts: Focus on areas you find challenging. Spend more time on those topics, but ensure you revisit easier areas to maintain balance.
- Use a Timer: Use the Pomodoro technique or set time limits for each study session to stay focused and avoid burnout.
Active Learning Techniques
Passive reading is often ineffective for deep learning. Engaging with the material actively will strengthen your understanding and memory retention.
- Practice Problem-Solving: Apply the concepts to real-life examples or practice questions. The more you apply what you’ve learned, the stronger your grasp on the material will be.
- Teach What You’ve Learned: Explaining concepts to others is a great way to reinforce your understanding. If you can teach it clearly, you’ve mastered it.
- Use Visual Aids: Diagrams, charts, and mind maps can help you visualize relationships between ideas and improve recall.
By incorporating these approaches, you’ll be better prepared and more confident in your abilities. Consistent practice, strategic planning, and active engagement with the content will lead to improved comprehension and better results in the long run.
- Molarity Formula: M = n / V