
Throughout history, scientists have worked tirelessly to uncover the fundamental composition of matter, seeking to understand how it behaves at the most basic level. Over time, theories have evolved, shifting from simple concepts to more intricate ideas that challenge our understanding of the universe.
In this section, we explore the key theories that have shaped our knowledge of particles, energy, and their interactions. Each breakthrough has led to new perspectives, revealing how tiny components form complex structures and give rise to the world we experience. Scientific advancements continue to refine these ideas, making them essential for anyone looking to grasp the underlying principles of physical science.
By examining these concepts, we can gain a deeper understanding of how matter behaves and how our scientific knowledge continues to progress. The insights provided by these theories have paved the way for modern discoveries in fields such as chemistry, physics, and materials science. Exploring these ideas allows us to appreciate the complexity and elegance of nature’s design.
Understanding Atomic Models
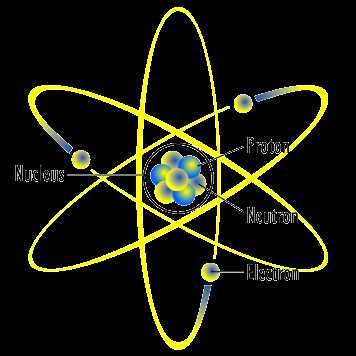
The study of matter’s fundamental structure has captivated scientists for centuries. As research progressed, different ideas emerged to explain how particles interact and form the foundation of all substances. These conceptual frameworks have undergone continuous transformation, each offering new insights into the intricate organization of matter.
At the core of scientific exploration lies the need to explain how particles such as electrons, protons, and neutrons behave within different environments. Over time, various theories have attempted to illustrate this behavior and predict outcomes of experiments, making these frameworks crucial for advancing scientific knowledge. Below are key developments that shaped our current understanding:
- Classical approaches: Early scientists envisioned matter as indivisible units, leading to initial atomic theories.
- Development of subatomic particles: Discoveries of protons, neutrons, and electrons led to the refinement of these ideas.
- Quantum mechanics: New perspectives emerged with the introduction of wave-particle duality, challenging classical notions.
- Energy levels: The arrangement of particles in specific energy states played a key role in understanding behavior and reactions.
These frameworks not only provide a foundational understanding but also offer valuable applications in modern technology, from semiconductors to medical imaging. Each theoretical shift has helped build a clearer picture of how even the smallest components of matter influence larger systems.
Key Concepts of Atomic Structure

Understanding the basic components that make up matter is essential to grasping how substances interact and behave under various conditions. The core idea revolves around identifying and describing the tiny, yet incredibly important, parts that constitute every material around us. These building blocks are organized in ways that determine chemical properties, reactions, and physical characteristics.
The structure of these particles, including how they are arranged and interact, plays a pivotal role in explaining phenomena like energy transfer, bonding, and molecular behavior. Key ideas central to this understanding include:
- Subatomic particles: The discovery of fundamental components like protons, neutrons, and electrons revealed how matter is structured at its core.
- Electron configuration: The arrangement of electrons in discrete energy levels around a nucleus governs chemical reactivity and bonding behaviors.
- Nuclear charge: The positive charge within the center of an entity influences its overall stability and interactions with other particles.
- Quantum principles: Quantum mechanics provides the framework for understanding the behavior of particles at the smallest scales, where classical physics no longer applies.
These fundamental concepts form the foundation for various scientific disciplines, influencing everything from chemistry to material science and quantum physics. Grasping them is crucial for deeper exploration into how matter behaves and interacts in different environments.
Historical Development of Atom Models
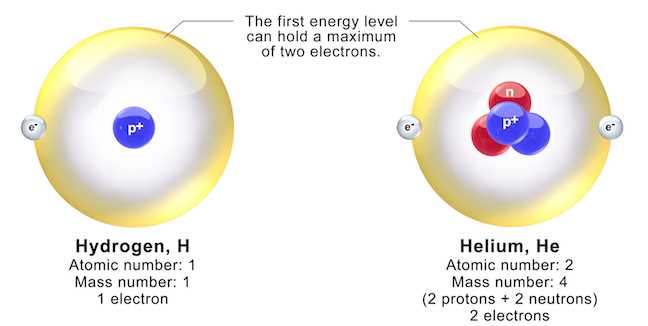
The quest to understand the basic components of matter has evolved over centuries, with theories and ideas gradually shifting to reflect new discoveries and technological advancements. Early concepts were simplistic, often relying on philosophical speculation, but as scientific methods improved, so too did the understanding of matter’s structure. Each breakthrough has contributed to the refinement of these theories, leading to a more accurate depiction of how particles interact and form substances.
Ancient Theories and Early Foundations
In ancient times, thinkers like Democritus proposed that matter was composed of indivisible units, which he called “atomos.” This idea, though speculative, laid the groundwork for later developments. For many centuries, however, it remained more of a philosophical notion than a scientific theory, as the tools needed to investigate matter at the subatomic level did not exist.
From Classical to Modern Understanding
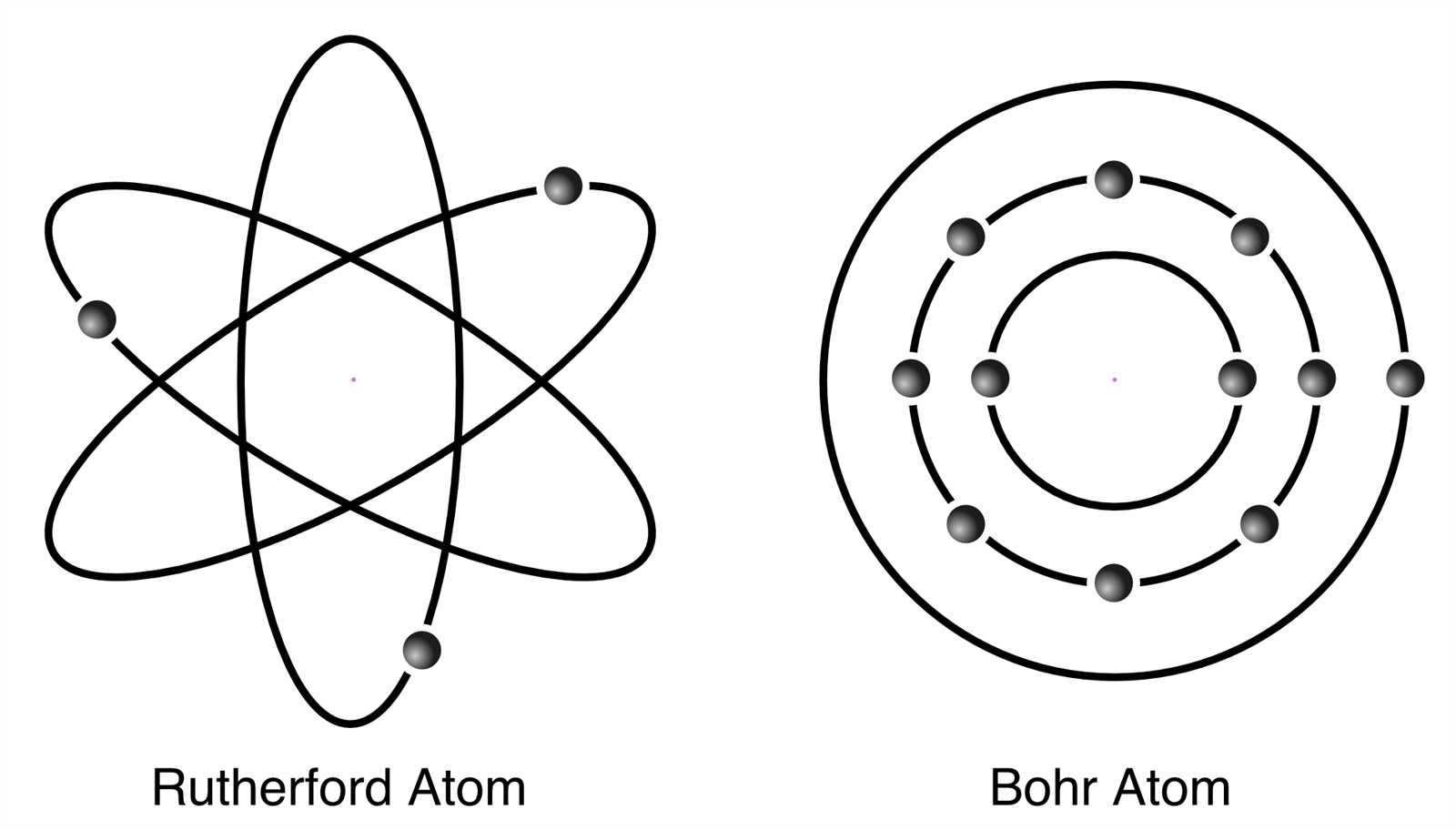
In the 19th and early 20th centuries, experiments and advancements in technology began to reveal more about matter’s inner workings. Scientists like Dalton, Thomson, Rutherford, and Bohr significantly altered the way we view matter’s structure. Dalton’s atomic theory proposed that each element consisted of identical particles, while Thomson’s discovery of electrons introduced the idea of subatomic components. Rutherford’s gold foil experiment revealed the existence of a dense nucleus, and Bohr’s work on energy levels helped explain electron behavior more accurately.
As these ideas continued to evolve, quantum mechanics emerged, offering a more comprehensive and precise understanding of particle behavior. These developments paved the way for modern atomic theory, where principles of uncertainty and wave functions govern our understanding of matter on the smallest scales.
Bohr Model and Its Significance
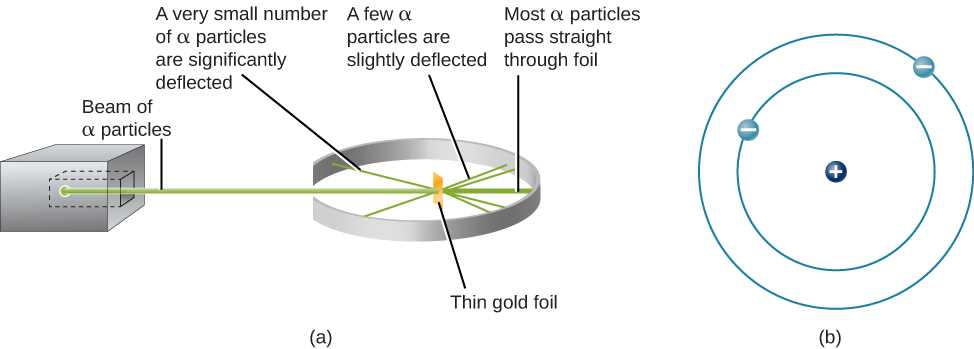
The concept introduced by Niels Bohr in the early 20th century revolutionized the understanding of how electrons are arranged and behave within a nucleus-centered system. Prior to Bohr’s theory, scientists struggled to explain certain observed phenomena, such as the emission spectra of elements. His work bridged gaps between classical physics and quantum theory, offering a clearer, more accurate picture of particle behavior.
Bohr proposed that electrons exist in distinct energy levels or orbits around the nucleus, with each level corresponding to a specific energy. These orbits were quantized, meaning electrons could only occupy certain allowed levels, and could absorb or emit energy only when transitioning between these levels. This idea successfully explained the spectral lines observed in hydrogen and laid the foundation for further advancements in atomic theory.
Beyond its immediate success in explaining atomic spectra, Bohr’s model marked a key shift in how scientists approached the study of matter. It provided a framework that not only enhanced the understanding of energy states but also opened the door for the development of quantum mechanics and more sophisticated theories of particle behavior. The Bohr model remains a critical milestone in the evolution of our understanding of the subatomic world.
Electron Orbits in the Atom
The arrangement of particles within a nucleus-centered system is a crucial aspect of understanding how elements interact and behave. Electrons, being negatively charged, are found at varying distances from the nucleus, with their position and movement governed by specific energy levels. These pathways, or orbits, are integral to explaining many properties of matter, such as chemical bonding and reactivity.
Electrons are not randomly distributed; instead, they occupy distinct energy levels or shells. Each shell corresponds to a specific energy, with electrons in lower energy levels being closer to the nucleus and those in higher levels located farther away. Key concepts related to electron orbits include:
- Energy quantization: Electrons can only exist in defined energy levels, and transitions between these levels result in energy absorption or emission.
- Electron configuration: The arrangement of electrons in different energy levels determines how an element will react with others.
- Stability and movement: Electrons in lower energy levels are more stable and less likely to leave the orbit compared to those in higher levels.
- Excited states: When electrons absorb energy, they can jump to higher levels, but they eventually return to their original state by emitting energy.
These principles help explain why elements have unique chemical properties and why they form bonds in specific ways. Understanding electron orbits is essential for grasping the behavior of materials at both the microscopic and macroscopic levels.
Quantum Mechanical Model of the Atom
In the early 20th century, advancements in quantum mechanics significantly altered our understanding of subatomic particles and their behavior. Unlike previous concepts that described particles in fixed orbits, this new framework introduced a probabilistic approach, where particles no longer have precise locations but are instead described by probability distributions. This theory has become a cornerstone in explaining the behavior of matter at the microscopic level.
Key Principles of Quantum Mechanics
The quantum mechanical description of matter is based on a few essential principles that differ greatly from classical physics. These ideas include:
- Wave-particle duality: Particles like electrons exhibit both wave-like and particle-like properties, influencing their behavior and interactions.
- Uncertainty principle: Introduced by Heisenberg, this principle states that it is impossible to know both the position and momentum of a particle with absolute certainty.
- Probability clouds: Rather than having fixed paths, particles are described by “clouds” of probability, indicating where they are likely to be found at any given moment.
Electron Distribution and Energy States
The quantum mechanical model provides a detailed understanding of how electrons are distributed in different energy states. These states are defined by specific quantum numbers that describe the size, shape, and orientation of electron clouds. The table below outlines these key quantum numbers:
| Quantum Number | Description |
|---|---|
| Principal Quantum Number (n) | Indicates the energy level of an electron, with higher values corresponding to higher energy and greater distance from the nucleus. |
| Azimuthal Quantum Number (l) | Describes the shape of the orbital (s, p, d, f) that an electron occupies. |
| Magnetic Quantum Number (ml) | Indicates the orientation of the orbital in space relative to the other orbitals. |
| Spin Quantum Number (ms) | Describes the intrinsic spin of an electron, either +1/2 or -1/2. |
These quantum numbers, along with the principles of wave-particle duality and uncertainty, help explain the behavior of particles in a way that classical physics could not. This model represents a leap forward in understanding, offering a more accurate and comprehensive framework for describing matter on the smallest scale.
Differences Between Classical and Quantum Models
Over the centuries, two major frameworks have shaped our understanding of matter at the subatomic level. The classical approach, which dominated until the early 20th century, relied on deterministic laws and fixed paths for particles. In contrast, the quantum approach introduced by early physicists presented a radically different view, emphasizing uncertainty, probability, and wave-like behavior. These two perspectives are fundamentally different in how they explain particle interactions, energy transfer, and the nature of reality at microscopic scales.
Key Distinctions in Assumptions

The primary differences between classical and quantum frameworks lie in their foundational assumptions. In classical theories, particles were believed to follow predictable, circular orbits with precise locations and speeds. Quantum theory, however, rejects this determinism, proposing that particles do not have definite positions or velocities until measured, and instead exist in probability distributions. Here are the main contrasts:
- Determinism vs. Probability: Classical mechanics assumes that knowing initial conditions allows for the prediction of future states. Quantum mechanics, however, provides probabilities, not certainties, for where particles might be found.
- Continuous vs. Discrete Energy: In classical models, energy changes occur continuously. In quantum models, energy is quantized, meaning it can only take specific discrete values.
- Pathways vs. Uncertainty: Classical models depict electrons as moving along fixed paths. Quantum mechanics, influenced by the uncertainty principle, suggests that the position and momentum of particles cannot both be precisely known at the same time.
Implications on Scientific Understanding
These differences have profound effects on how scientists approach and interpret phenomena. Classical mechanics is highly effective for describing macroscopic systems and everyday objects, where quantum effects are negligible. However, when examining very small particles, such as electrons, the predictions of classical physics fail. Quantum theory has provided accurate models for understanding atomic spectra, chemical bonding, and particle interactions, challenging long-held views and expanding our understanding of the universe.
The Role of Subatomic Particles

Subatomic particles are fundamental components that define the properties and behavior of matter. These tiny particles interact in complex ways, giving rise to the structure and characteristics of all substances. Understanding their roles and relationships is crucial for explaining everything from chemical reactions to the behavior of light and energy at the smallest scales.
Within every system, these particles–electrons, protons, and neutrons–interact with one another to form the building blocks of elements and compounds. The arrangement and behavior of these particles influence the chemical properties, stability, and reactivity of matter. Each particle plays a specific role:
- Electrons: Negatively charged particles that orbit around the nucleus, their distribution in various energy levels determines the chemical properties and bonding behavior of an element.
- Protons: Positively charged particles located in the nucleus, determining the identity of an element through the atomic number and contributing to the mass of the nucleus.
- Neutrons: Neutral particles also found in the nucleus, playing a key role in the stability of the nucleus and affecting the isotopic form of an element.
The interactions between these subatomic particles are governed by fundamental forces, including the electromagnetic and nuclear forces, which dictate the behavior of matter at both macroscopic and microscopic levels. The study of these interactions continues to be at the forefront of scientific research, providing deep insights into the nature of the universe and the forces that shape it.
Rutherford’s Contribution to Atomic Theory
In the early 20th century, groundbreaking experiments challenged the existing understanding of matter and its structure. One of the key figures in reshaping this theory was Ernest Rutherford, whose work revealed surprising insights into the composition and behavior of matter at its most fundamental level. His discoveries not only advanced scientific thought but also paved the way for future research in nuclear physics and chemistry.
Gold Foil Experiment
Rutherford’s most famous experiment, known as the gold foil experiment, involved bombarding a thin sheet of gold foil with alpha particles. The results of this experiment led to a dramatic shift in the way scientists viewed the internal structure of matter. Contrary to the prevailing “plum pudding” model, which suggested that positive charge was evenly spread out within an atom, Rutherford observed that most alpha particles passed through the foil, but a small fraction were deflected at large angles. This led him to conclude that most of the mass of an atom is concentrated in a tiny, dense nucleus at its center, while the rest of the atom is mostly empty space.
Impact on Atomic Theory

Rutherford’s findings led to the development of the nuclear model of matter, which proposed that atoms consist of a central nucleus, containing positively charged protons, surrounded by negatively charged electrons. This marked a significant departure from earlier ideas and laid the foundation for later atomic theories, including Bohr’s model. Rutherford’s discovery not only advanced our understanding of atomic structure but also sparked further research into the properties of subatomic particles and the forces that govern them.
Understanding Atomic Spectra
When matter absorbs or emits energy, it often produces specific patterns of light known as spectra. These patterns provide key insights into the internal structure and energy levels of individual particles. By studying these spectra, scientists are able to understand how energy interacts with particles and gain deeper knowledge of their properties.
Each element emits or absorbs light at characteristic wavelengths, creating a unique spectral signature. This occurs when electrons within particles move between different energy levels, releasing or absorbing energy in the form of photons. The resulting light can be split into a spectrum, which serves as a fingerprint for each element. These spectra are typically classified into two categories: emission and absorption spectra.
- Emission Spectra: Produced when particles release energy as light as electrons drop from higher to lower energy states.
- Absorption Spectra: Formed when electrons absorb energy and move to higher energy states, leaving dark lines in the spectrum.
The study of atomic spectra has played a vital role in advancing quantum theory and understanding the behavior of electrons within particles. These spectral patterns help confirm the quantized nature of energy levels and offer valuable tools for identifying and analyzing substances in fields such as chemistry and astronomy.
Wave-Particle Duality of Electrons
The nature of matter has been a subject of intense debate and exploration, particularly when it comes to understanding how particles, like electrons, behave. Traditionally, particles were thought to have specific, localized positions, while waves were thought to spread out and propagate through space. However, modern physics has revealed that subatomic particles, such as electrons, exhibit both wave-like and particle-like properties. This phenomenon is known as wave-particle duality.
Wave-particle duality implies that electrons can behave as discrete particles under certain conditions, yet they also display wave-like characteristics, such as interference and diffraction, under other circumstances. This concept challenges our classical understanding of the physical world and is a cornerstone of quantum mechanics.
| Property | Wave Behavior | Particle Behavior |
|---|---|---|
| Nature | Continuous, spread out | Discrete, localized |
| Examples | Diffraction, interference | Collisions, energy transfer |
| Explanation | Described by wavelength and frequency | Described by mass and velocity |
This dual behavior was first demonstrated in experiments such as the double-slit experiment, where electrons, when passed through two slits, created an interference pattern typically associated with waves. However, when observed directly, electrons behaved as individual particles. This paradox, which lies at the heart of quantum mechanics, continues to shape our understanding of the microscopic world and challenges classical physics concepts.
Heisenberg’s Uncertainty Principle Explained
In the realm of quantum mechanics, one of the most profound and puzzling concepts is the inherent uncertainty in measuring certain properties of particles. This idea was introduced by Werner Heisenberg, and it suggests that there are fundamental limits to how precisely we can simultaneously know certain characteristics of subatomic particles, such as position and momentum. This principle challenges classical notions of predictability and determinism, revealing a core aspect of quantum behavior.
The Core Idea
Heisenberg’s uncertainty principle states that the more precisely one property of a particle, such as its position, is measured, the less precisely another property, such as its momentum, can be known. This is not a limitation of measurement tools, but rather a fundamental feature of nature itself. The principle highlights the intrinsic fuzziness of the quantum world, where exact values for multiple properties cannot be simultaneously determined with absolute certainty.
Mathematical Expression
The uncertainty principle is mathematically expressed as follows:
Δx * Δp ≥ h / 4π
Where Δx represents the uncertainty in position, Δp represents the uncertainty in momentum, and h is Planck’s constant. This inequality shows that there is a limit to how precisely both position and momentum can be known at the same time. The smaller the uncertainty in one property, the greater the uncertainty in the other.
This principle has far-reaching implications, not only in physics but in how we understand the nature of reality at the quantum level. It suggests that at a fundamental level, the universe does not operate with perfect precision, but with a degree of unpredictability that shapes the behavior of particles and systems.
Schrodinger’s Equation and Its Impact
One of the cornerstones of modern quantum mechanics is an equation that describes how physical systems evolve over time. Erwin Schrödinger’s wave equation provides a mathematical framework to understand the behavior of particles, particularly at microscopic scales. Unlike classical mechanics, which can predict exact paths for particles, this equation deals with probabilities, offering insights into the likely locations and behaviors of particles in various states.
The Wave Function
At the heart of Schrödinger’s equation lies the concept of the wave function, a mathematical tool that encodes all the possible information about a system. The wave function, often represented by the Greek letter Ψ (Psi), is a complex function that varies with both position and time. The square of its absolute value, |Ψ|², gives the probability density, which describes the likelihood of finding a particle in a specific location.
Mathematical Formulation

The time-dependent Schrödinger equation is written as:
iħ ∂Ψ/∂t = HΨ
Where i is the imaginary unit, ħ is the reduced Planck constant, ∂Ψ/∂t is the time derivative of the wave function, and H is the Hamiltonian operator that represents the total energy of the system.
| Component | Description |
|---|---|
| Ψ (Wave Function) | Describes the quantum state of the system. |
| H (Hamiltonian Operator) | Represents the total energy of the system (kinetic + potential). |
| ħ (Reduced Planck Constant) | Fundamental constant relating energy and frequency. |
| i (Imaginary Unit) | Used to express complex numbers in the equation. |
Schrödinger’s equation revolutionized the understanding of quantum systems by introducing a framework that goes beyond classical trajectories. Instead of determining exact positions and velocities, the equation provides a probabilistic model for the behavior of particles. This shift in perspective has had profound implications for fields ranging from chemistry to electronics, allowing scientists to predict the behavior of atoms and molecules with remarkable accuracy.
In essence, Schrödinger’s work opened the door to a deeper understanding of matter at the quantum level, making it possible to explore the microscopic world with mathematical precision. Its influence continues to shape scientific research and technological advancements today.
Modern Atomic Theory Applications
In recent years, the principles derived from advanced quantum mechanics have found applications in various fields, transforming technology and science. By understanding the intricate behavior of particles on a subatomic level, researchers have been able to develop tools and solutions that were once thought impossible. These insights are not just limited to theoretical physics; they have tangible impacts in industries ranging from medicine to information technology.
Below are some of the key areas where modern atomic concepts are applied:
- Semiconductors and Electronics: Quantum mechanics plays a crucial role in the design and operation of semiconductor devices. Technologies such as transistors and microchips rely heavily on principles derived from atomic structure and behavior.
- Medical Imaging: Techniques such as MRI (Magnetic Resonance Imaging) and PET (Positron Emission Tomography) are based on the understanding of atomic interactions with electromagnetic fields. These imaging methods help diagnose and treat various diseases.
- Nuclear Energy: Modern atomic theory underpins the understanding of nuclear reactions, which are essential for the development and management of nuclear power plants. The principles also extend to radiation therapy for cancer treatment.
- Quantum Computing: Emerging technologies in computing depend on the ability to manipulate particles at the quantum level. By leveraging quantum superposition and entanglement, quantum computers have the potential to solve complex problems faster than classical computers.
- Laser Technology: The operation of lasers, which have applications in fields ranging from telecommunications to surgery, is fundamentally grounded in the quantum behavior of electrons and photons.
As research in quantum mechanics and atomic theory progresses, new applications are continually being discovered. These advances not only push the boundaries of existing technologies but also open up exciting possibilities for future innovations. Understanding the behavior of particles on such a small scale continues to be a driving force for breakthroughs across multiple disciplines.
Key Experiments in Atomic Research
Throughout history, groundbreaking experiments have shaped our understanding of matter at its most fundamental level. These key investigations provided the evidence and insights that challenged existing theories and introduced new perspectives on how particles behave. Each discovery not only advanced the field but also paved the way for the technologies we use today. Below are some of the most influential experiments in this area:
- Rutherford’s Gold Foil Experiment: Conducted in 1909, this experiment led to the discovery of the nucleus. By firing alpha particles at a thin gold foil, Rutherford observed that most particles passed through, but some were deflected, indicating the presence of a dense, positively charged center within the atom.
- Thomson’s Cathode Ray Tube Experiment: In the late 19th century, J.J. Thomson used a cathode ray tube to investigate the properties of electric currents in gases. This experiment led to the discovery of the electron, demonstrating that atoms contain smaller, negatively charged particles.
- Planck’s Blackbody Radiation Experiment: Max Planck’s work in the early 1900s helped lay the foundation for quantum theory. His study of blackbody radiation revealed that energy is emitted in discrete units, or “quanta,” fundamentally altering our understanding of energy and matter.
- Bohr’s Hydrogen Spectrum Experiment: Niels Bohr’s work on the hydrogen atom helped establish the concept of quantized energy levels. By studying the spectral lines of hydrogen, he proposed that electrons orbit the nucleus in fixed paths, emitting or absorbing energy in discrete amounts.
- Heisenberg’s Uncertainty Principle: Werner Heisenberg formulated the uncertainty principle, which states that it is impossible to simultaneously know both the position and momentum of a particle with absolute precision. This principle revolutionized the way scientists view particle behavior at a quantum level.
These experiments, along with many others, have expanded our knowledge of how matter is structured and how particles interact. Each experiment challenged preconceived notions and brought new insights into the behavior of matter at the microscopic level, influencing various fields of science and technology to this day.