
The movement of substances across cell membranes is a fundamental concept in understanding how living organisms function. Through controlled experiments, students explore the factors that influence how materials like water and nutrients pass into and out of cells. This section provides essential insights into these processes, helping to clarify the principles that govern cellular interactions with their environment.
In this article, we will examine the results of practical exercises that focus on the mechanisms of material transfer, highlighting the roles that concentration gradients, temperature, and pressure play in these natural processes. By reviewing the outcomes of such experiments, learners can deepen their understanding of the conditions under which specific behaviors occur.
After completing the exercises, students are encouraged to compare their findings with the provided analysis to ensure accurate interpretations. This approach allows for a better grasp of the core concepts involved, reinforcing the connection between theory and real-world applications.
AP Biology Investigation 4 Diffusion and Osmosis Answer Key
This section provides an overview of the essential concepts explored in the experiment focused on cellular transport processes. The experiment aims to shed light on how substances move across membranes, a crucial aspect of cellular function. By observing the behavior of particles in various solutions, students gain a deeper understanding of the factors that influence material transfer within living systems.
Through the hands-on approach of the experiment, learners investigate how different variables, such as concentration gradients and environmental conditions, affect the movement of molecules. The goal is to recognize patterns and understand the mechanisms that govern material flow in biological contexts. Understanding these processes is fundamental to comprehending how cells maintain balance and function efficiently.
By reviewing the results and comparing them with the provided analysis, students are able to verify their findings and develop a more accurate interpretation of the observed phenomena. This process helps reinforce the connection between theoretical knowledge and practical application, offering a comprehensive understanding of the subject matter.
Understanding Diffusion and Osmosis Principles
The movement of substances across the boundary of a cell is a fundamental process that ensures proper functioning and survival. These processes are driven by the tendency of materials to move from areas of high concentration to areas of low concentration, a phenomenon that plays a vital role in maintaining cellular homeostasis. By understanding the principles behind these movements, one can better grasp how cells interact with their environment and regulate internal conditions.
Key Factors Influencing Material Movement
Several factors contribute to the efficiency and rate of movement of substances through membranes. Concentration gradients, temperature, and the permeability of the membrane are the primary influences. The higher the concentration difference across a membrane, the faster the materials will tend to move. Additionally, temperature changes can affect molecular motion, influencing the rate at which materials pass through cellular barriers.
| Factor | Effect on Movement |
|---|---|
| Concentration Gradient | The greater the difference, the faster the movement |
| Temperature | Higher temperatures increase molecular speed and movement |
| Membrane Permeability | A more permeable membrane allows faster transfer |
Types of Material Movement Across Membranes
There are various ways materials move through cellular membranes, but the most common mechanisms are passive transport, where no energy is required, and active transport, where the cell expends energy to move substances. Understanding how these mechanisms operate under different conditions is essential for comprehending how cells maintain their internal environment and perform necessary functions.
Key Concepts in Cell Membrane Transport
The movement of substances across the cell membrane is essential for maintaining cellular function and homeostasis. This process is influenced by various factors, including the properties of the membrane itself and the nature of the substances being transported. Understanding these concepts is critical for grasping how cells interact with their environment, exchange materials, and regulate internal conditions.
Passive Transport Mechanisms
In passive transport, molecules move across the membrane without the need for energy input. This occurs as substances travel down their concentration gradient, from areas of high concentration to areas of low concentration. This process includes methods such as simple diffusion, facilitated diffusion, and the movement of water through specialized channels.
Active Transport Processes
Unlike passive transport, active transport requires energy to move substances against their concentration gradient. This is accomplished through membrane proteins known as pumps, which use cellular energy to move molecules from areas of low concentration to areas of high concentration. This mechanism is essential for maintaining cellular balance and supporting processes like nutrient uptake and waste removal.
Overview of AP Biology Investigation 4
This section explores an essential experiment focused on the movement of substances across cellular membranes. The goal is to better understand how molecules interact with cells, how they move in response to various factors, and how this movement impacts the function of living organisms. The experiment involves examining different types of material transport and the conditions under which these processes occur.
During the experiment, students observe how different factors influence the movement of molecules across a membrane, such as concentration gradients, temperature, and the nature of the materials involved. The results provide insights into how cells regulate their internal environment and maintain balance.
Objectives of the Experiment
- Understand the principles governing substance movement through membranes
- Investigate the impact of concentration differences on material transfer
- Examine the role of temperature in altering transport rates
- Analyze real-life examples of membrane transport in living cells
Materials and Methods
The experiment typically involves simple materials such as semipermeable membranes, water, and various solute solutions. The setup allows for the observation of material movement under controlled conditions. By adjusting key variables like concentration and temperature, students can visualize how substances pass through barriers.
Expected Outcomes
- Observation of material movement from high to low concentration areas
- Understanding how temperature affects the speed of transport
- Identifying the role of different types of solutions in membrane permeability
Factors Affecting Diffusion Rates
The rate at which materials move across membranes is influenced by several key factors. These factors determine how quickly molecules will travel from one area to another, playing a vital role in the efficiency of cellular processes. Understanding these variables is crucial for analyzing how cells exchange substances with their environment and maintain internal balance.
Concentration Gradient
The concentration difference between two areas is one of the primary drivers of material movement. A steeper gradient, where there is a larger difference in concentration between two regions, generally leads to faster movement. The greater the imbalance, the quicker particles tend to move in order to reach equilibrium.
Temperature
Temperature has a significant impact on the movement of particles. As temperature increases, the kinetic energy of molecules also rises, causing them to move more rapidly. This results in a higher rate of transport, as molecules collide and disperse more efficiently when heated.
Membrane Permeability
The ability of a membrane to allow substances to pass through also affects how quickly materials can move. Membranes with higher permeability enable faster transport, while those with lower permeability restrict the flow of molecules, slowing the overall rate of movement.
Exploring Osmosis in Biological Systems
The movement of water across cell membranes is a fundamental process that affects the overall function of living organisms. This type of transport is crucial for maintaining cellular hydration, nutrient balance, and waste removal. Understanding how water molecules move in response to concentration differences provides insight into how cells manage their internal environments and adapt to changes in their surroundings.
Water Movement in Different Environments
Water moves through membranes based on the concentration of solutes in the surrounding environment. When a cell is placed in a solution with a higher solute concentration than its internal environment, water moves out of the cell to balance the concentrations. Conversely, when the surrounding solution has a lower solute concentration, water enters the cell. This balance is essential for maintaining cell integrity and function.
Role of Water in Cell Function
Water is essential for various cellular processes, from nutrient transport to enzyme function. The flow of water into or out of cells influences the shape, size, and overall health of the cell. This process helps cells maintain turgor pressure, which is particularly important in plant cells for maintaining structural integrity.
Key Concept: The movement of water is not only crucial for survival but also plays a role in processes like plant growth, kidney filtration, and maintaining blood pressure in animals. Understanding how water behaves within biological systems offers valuable insights into the intricate mechanisms that keep living organisms functioning properly.
Types of Solutions in Osmosis Experiments
In experiments examining the movement of water across membranes, the surrounding solution plays a crucial role in determining how substances move into or out of cells. The nature of the solution can affect the direction and rate of movement, making it essential to understand the different types of environments that can be used in these experiments. Solutions are typically classified based on their solute concentration relative to the cell’s internal environment.
Hypertonic Solutions
In a hypertonic solution, the concentration of solutes outside the cell is higher than inside. As a result, water tends to move out of the cell to balance the concentrations, often causing the cell to shrink or lose volume. This type of solution can lead to dehydration in cells and is commonly observed in experiments testing the effects of high solute environments.
Hypotonic Solutions
In contrast, a hypotonic solution has a lower concentration of solutes compared to the inside of the cell. Water moves into the cell, potentially causing it to swell. If the intake of water is excessive, the cell may burst, especially in environments lacking a rigid cell wall, like animal cells. This type of solution is often used to study the effects of water influx on cell size and integrity.
Isotonic Solutions
An isotonic solution has an equal concentration of solutes inside and outside the cell. In this environment, there is no net movement of water into or out of the cell, resulting in a stable cell size. Isotonic conditions are important in experiments that aim to observe cellular activity without the confounding effects of water movement.
How Concentration Gradients Influence Movement
The movement of substances across cell membranes is largely driven by differences in concentration. These differences create a natural tendency for molecules to move from regions of high concentration to areas of lower concentration. The process is influenced by the gradient, which is the difference in the concentration of a substance across a membrane. A steeper gradient results in faster movement, while a gentler gradient slows it down.
Factors Affecting the Gradient
- Concentration Difference: A larger difference in concentration between two areas leads to a stronger gradient, increasing the speed of material movement.
- Temperature: Higher temperatures typically increase the movement of particles, causing a faster adjustment to equilibrium.
- Distance: The greater the distance over which a substance must move, the slower the rate of movement, even if the gradient is steep.
Impact on Cellular Processes
The concentration gradient plays a critical role in maintaining homeostasis within cells. For example, cells use gradients to regulate nutrient intake, remove waste products, and maintain internal pressure. In the case of cells exposed to external solutions, the gradient can influence the speed at which molecules enter or leave the cell, directly affecting cellular function.
Practical Applications of Diffusion and Osmosis
The movement of molecules across cell membranes is a fundamental process that has far-reaching applications in both biological systems and technology. From regulating the intake of nutrients in living organisms to the design of medical treatments and industrial processes, understanding these mechanisms is key to solving a variety of challenges. The principles governing this movement are applied in diverse fields ranging from healthcare to food preservation.
Medical Applications
Dialysis: One of the most significant medical applications of selective permeability is in dialysis treatments for patients with kidney failure. Dialysis machines use a semipermeable membrane to filter waste and excess fluids from the blood, mimicking the natural process of filtration in healthy kidneys.
Drug Delivery: The controlled release of drugs often depends on the ability of molecules to move through membranes. In pharmaceutical applications, understanding how substances pass through barriers enables the design of effective drug delivery systems that ensure consistent and targeted release into the bloodstream.
Food Preservation
Salting and Curing: In food processing, salt is often used to preserve meat and fish. The high concentration of salt in the external environment draws out water from the food through selective permeability, inhibiting the growth of bacteria and fungi that thrive in moist conditions.
Fruit Ripening: The process of ripening in fruits involves the movement of gases and nutrients. For example, ethylene gas plays a crucial role in the ripening of fruits, and controlling the movement of gases can help extend shelf life during transportation and storage.
Environmental Impact
Understanding how substances move through water and air is also essential in managing pollution and designing environmental cleanup strategies. Techniques such as reverse osmosis are used to purify water by removing contaminants, improving water quality for human consumption and agriculture.
Common Mistakes in Osmosis Experiments
When conducting experiments involving the movement of water through membranes, several common errors can lead to inaccurate results. These mistakes often arise from improper experimental design, handling of materials, or misinterpretation of observations. By understanding these frequent pitfalls, it becomes easier to conduct reliable experiments and obtain meaningful data.
Errors in Experimental Setup
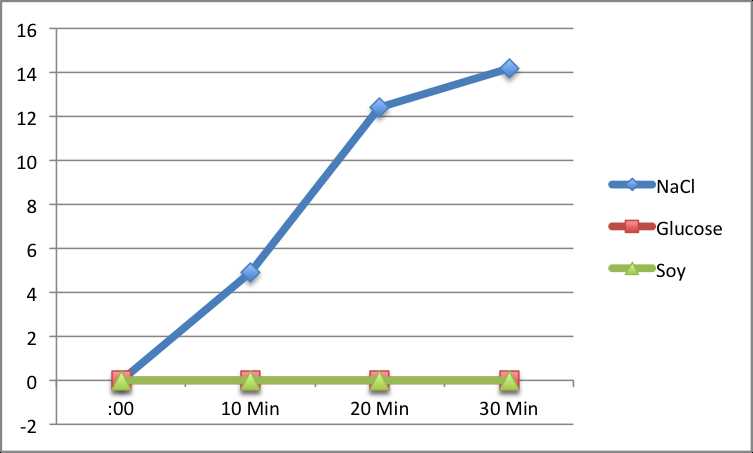
- Improper Solution Concentration: Using incorrect concentrations of solute can lead to misleading results. If the solution concentration is not measured accurately, the rate of water movement may not be as expected.
- Inconsistent Sample Size: Using different sizes of cells or membranes in experiments can skew results, as the rate of water movement is influenced by the surface area and volume of the sample.
- Temperature Variability: Conducting experiments at varying temperatures without proper controls can alter the movement of molecules, leading to inconsistent data. Temperature directly affects the rate of molecular motion, influencing results.
Handling and Measurement Mistakes
- Poor Sealing of Membranes: Inaccurate sealing of the experimental membrane can allow substances to pass through in ways that do not reflect the intended setup, causing unreliable results.
- Improper Timing: Failing to measure the duration of the experiment accurately can lead to misinterpretation of how long the process took and the extent of movement that occurred.
- Incorrect Measurement of Water Loss or Gain: Not measuring the change in mass or volume carefully can lead to incorrect conclusions about the rate of movement. Accurate weighing or measuring tools are essential for reliable data.
Misinterpretation of Results
- Overlooking Environmental Factors: Environmental factors, such as air currents or light exposure, can inadvertently influence results. Not accounting for these can lead to skewed data.
- Confusing Direction of Movement: A common mistake is misinterpreting the direction of water movement, especially when dealing with complex experimental setups. Clear observation and understanding of how water moves in response to solute concentration are essential.
Analyzing Results from Investigation 4
After conducting experiments that involve the movement of substances across membranes, analyzing the results is crucial to understanding the underlying processes. The data collected from these experiments can reveal patterns, help verify hypotheses, and highlight areas for further study. Proper analysis allows for a deeper understanding of how environmental conditions and solute concentrations affect molecular movement.
Steps to Analyze the Results
- Organizing Data: Start by organizing the collected data into clear, structured tables or graphs. This allows for easier comparison between different trials and conditions.
- Identifying Trends: Look for trends in the data. Are there noticeable patterns, such as faster movement with higher concentrations of solute or greater rates of change in smaller samples?
- Comparing Experimental Groups: Compare the results from different experimental groups. This can help determine if variables such as concentration, temperature, or sample size influenced the outcome in a predictable way.
- Statistical Analysis: Use statistical methods, such as calculating averages, standard deviations, or conducting t-tests, to assess the significance of your results.
Common Observations to Consider
- Rate of Movement: The rate at which substances move across the membrane can provide insight into the effects of concentration gradients and the selective permeability of the membrane.
- Changes in Mass or Volume: Analyzing changes in mass or volume of the samples before and after the experiment can give clues about the extent of molecular movement, whether it is water, ions, or other substances.
- Unexpected Results: If the data does not align with expected outcomes, consider possible errors in the experimental setup or measurement. This may point to areas that need further refinement.
Interpreting Diffusion and Osmosis Data
When examining data from experiments that explore the movement of molecules across membranes, it is essential to carefully interpret the results to draw meaningful conclusions. Data analysis can reveal how certain factors, such as concentration gradients, temperature, and membrane permeability, influence the movement of substances. Proper interpretation helps in understanding the principles governing these processes and provides insights into the efficiency and accuracy of the experimental setup.
In order to analyze the data effectively, it is important to focus on key variables and compare how they impact the outcomes. By examining trends and patterns, researchers can better understand the relationship between factors and molecular movement. The following table outlines typical results observed in these experiments and their possible implications.
| Condition | Expected Outcome | Interpretation |
|---|---|---|
| Higher solute concentration | Faster movement of water or molecules | Indicates a stronger concentration gradient, promoting greater movement of molecules across the membrane. |
| Lower temperature | Slower rate of movement | Lower temperatures reduce the kinetic energy of molecules, leading to a slower rate of molecular motion. |
| Smaller membrane surface area | Slower movement | Decreased surface area limits the available space for molecular transport, reducing the rate of movement. |
| Increased membrane permeability | Faster movement | More permeable membranes allow for quicker passage of molecules, resulting in faster movement across the barrier. |
Understanding these trends is key to accurately interpreting the data. Any discrepancies between expected and observed results should be carefully analyzed to identify potential sources of error or unaccounted variables. By reviewing these factors systematically, researchers can ensure a comprehensive understanding of the process under investigation.
Visualizing Osmosis with Real-World Examples

Understanding the movement of molecules across membranes becomes more tangible when it is related to everyday occurrences. Real-world examples provide a clear and relatable way to grasp the principles behind molecular transport, helping to bridge the gap between theoretical knowledge and practical application. By observing common scenarios, one can see how these processes play a critical role in various biological systems and everyday phenomena.
Several real-world examples help illustrate how this natural movement occurs in various environments. Below are a few situations where molecular movement can be easily observed:
- Salt and Cucumber – When a cucumber is placed in salt, water from the cells moves out, causing the cucumber to shrink. This is an example of water moving out of a high-concentration area inside the cucumber’s cells to a lower concentration in the surrounding salt solution.
- Kidneys Filtering Blood – In the human body, kidneys filter waste from the blood. This process relies on the movement of water and solutes across membranes to maintain balance and remove excess substances from the bloodstream.
- Plants Absorbing Water – Plants absorb water from the soil through their roots, where water moves from an area of low solute concentration in the soil to a higher concentration inside the plant’s roots, helping them maintain hydration and nutrient intake.
- Raisins in Water – When dried raisins are placed in water, they swell as they absorb water. This happens because the concentration of water outside the raisin is higher, leading to the movement of water into the raisin’s cells.
Each of these examples highlights how movement occurs based on the concentration differences between the inside of a cell or structure and the surrounding environment. Observing these processes in real-life scenarios helps to better understand the science behind them, making the concept more accessible and easier to visualize.
Comparing Hypertonic, Hypotonic, and Isotonic Solutions
In order to understand how cells interact with their environments, it’s important to recognize the differences between various types of solutions that can surround cells. These solutions differ in their concentration of solutes relative to the inside of the cell, which influences the movement of water and other molecules. Understanding these differences is key to exploring how substances move across membranes and how cells maintain balance.
When comparing solutions, there are three main types to consider: hypertonic, hypotonic, and isotonic. Each of these has distinct characteristics that affect cellular processes in unique ways:
- Hypertonic Solutions – A hypertonic solution has a higher concentration of solutes compared to the inside of a cell. When a cell is placed in such a solution, water moves out of the cell to balance the concentrations, causing the cell to shrink or lose volume.
- Hypotonic Solutions – In a hypotonic solution, the concentration of solutes is lower outside the cell than inside. As a result, water moves into the cell, causing it to swell or even burst if the imbalance is too extreme.
- Isotonic Solutions – An isotonic solution has the same concentration of solutes as the inside of the cell. In this case, water moves in and out of the cell at an equal rate, keeping the cell’s size and shape stable.
These differences have significant implications for the health and function of cells. In a hypertonic environment, cells lose water and can become dehydrated. In a hypotonic environment, cells may swell excessively and potentially burst. In an isotonic environment, cells remain stable, maintaining proper hydration and function.
Cellular Responses to Osmotic Pressure
Cells are constantly exposed to changes in their surrounding environments, which can vary in terms of solute concentrations. These fluctuations create osmotic pressure, which is the force that drives the movement of water across cell membranes. The cell must respond to this pressure in ways that help maintain homeostasis and prevent damage or dysfunction. The way a cell reacts to osmotic pressure is crucial for its survival and proper functioning.
When cells are exposed to varying levels of osmotic pressure, they can adopt several mechanisms to manage water balance. The response largely depends on the surrounding solution and the cell’s environment:
- In Hypertonic Environments – When a cell is surrounded by a solution with higher solute concentration, water tends to leave the cell. In response, the cell may shrink, a process known as plasmolysis. In plants, the cell membrane pulls away from the cell wall, while in animal cells, dehydration occurs, potentially leading to cellular damage.
- In Hypotonic Environments – In contrast, if the external solution has a lower concentration of solutes, water moves into the cell, causing it to swell. Animal cells may burst if the pressure becomes too great, a phenomenon known as lysis. Plant cells, however, usually manage this situation better due to the presence of a rigid cell wall that prevents them from bursting, although they can still experience excessive turgor pressure.
- In Isotonic Environments – When the solute concentration inside and outside the cell is equal, there is no net movement of water. This balance maintains the cell’s size and shape, allowing it to function optimally without the stress of excess pressure in either direction.
The ability of cells to regulate water movement through various mechanisms is essential for maintaining their integrity. These responses help cells cope with osmotic pressure changes and avoid harmful consequences like dehydration, swelling, or rupture, ensuring that they can thrive in diverse environmental conditions.
Significance of Controlled Experiment Variables
In scientific experimentation, ensuring the reliability and accuracy of results relies heavily on controlling specific factors. By carefully managing variables, researchers can isolate the effects of the variable of interest and draw clear, valid conclusions. Without proper control, external factors could introduce errors or inconsistencies, making it difficult to understand the true relationship between variables. A well-designed experiment involves maintaining constant conditions, allowing for a more precise evaluation of how one factor influences another.
Importance of Independent and Dependent Variables

The distinction between independent and dependent variables is foundational in experimental design. The independent variable is the one that is deliberately manipulated to observe its effect on the dependent variable, which is measured. Maintaining consistency in all other factors ensures that any observed changes can be attributed to the manipulation of the independent variable.
Controlling Extraneous Variables
Extraneous variables are factors that are not of primary interest but can still affect the outcome of the experiment. For instance, environmental conditions such as temperature, light, or humidity can influence the behavior of cells or substances. To ensure the validity of results, researchers must control or account for these extraneous variables to minimize their potential impact.
Overall, controlling variables is crucial for obtaining reliable, reproducible results that reflect true cause-and-effect relationships. Effective control of variables ensures that experiments are designed with scientific rigor, providing a solid foundation for drawing meaningful conclusions and advancing knowledge.
Steps for Conducting Diffusion and Osmosis Experiments
Performing experiments that explore the movement of substances across membranes involves a systematic approach to ensure accurate results. These procedures typically require careful planning, preparation of materials, and precise measurements. A clear understanding of the process and the factors involved will help researchers draw meaningful conclusions about the interactions between substances in different environments. Below are the steps commonly followed in such experiments.
Preparation and Setup
The first step in any experiment is to gather all necessary materials and prepare the workspace. This includes ensuring that the appropriate substances and equipment are available and ready for use. Typical materials include:
- Semipermeable membranes
- Solutions with varying concentrations
- Containers (such as beakers or Petri dishes)
- Measuring instruments (e.g., scales, pipettes)
- Timer for measuring intervals
Experimental Procedure
Once the materials are ready, the next step involves setting up the experiment. This typically includes:
- Preparing different concentrations of solutes to observe the effects on substance movement.
- Placing the semipermeable membranes in the solutions and ensuring they are properly submerged.
- Monitoring the time intervals to observe any changes that occur, such as volume shift or substance transfer.
- Carefully recording data at each stage to track the results.
Data Collection and Analysis
During the experiment, data collection is crucial for understanding the results. After completing the experiment, the data should be analyzed to determine patterns and draw conclusions. Data points might include the movement of substances, changes in solution concentrations, or volume changes over time.
Conclusion and Reporting
After data analysis, the final step is to interpret the results in light of the original hypothesis. Understanding how substances interact with their environments helps clarify the principles behind their movement. Conclusions are typically reported through tables or graphs that visually represent the findings.
Below is an example of how the data from such an experiment might be presented:
| Time (min) | Concentration of Solution 1 | Concentration of Solution 2 | Change in Volume |
|---|---|---|---|
| 0 | 0.5M | 1.0M | 0 mL |
| 10 | 0.5M | 1.0M | +5 mL |
| 20 | 0.5M | 1.0M | +10 mL |
By following these steps, researchers can ensure that the experiment is carried out in a systematic manner, allowing for the collection of reliable and reproducible data.
Using the Answer Key to Clarify Results
Interpreting experimental results can be challenging, especially when data doesn’t align with initial expectations. In such cases, a solution reference guide can be extremely helpful in clarifying the meaning of the observations. By comparing results with established patterns or expected outcomes, it becomes easier to pinpoint where discrepancies might have occurred and why. This resource offers insights that allow for a deeper understanding of the processes at play.
Verifying Expected Outcomes
When analyzing experimental data, it’s important to have a reference point that outlines what the results should look like under controlled conditions. This guide can provide clarity on how certain variables should behave, helping to determine if the process occurred as expected. By matching your results with this reference, you can identify if any adjustments need to be made or if there was an error in the setup.
Correcting Misunderstandings
Sometimes, results can be misinterpreted due to incorrect assumptions or overlooked factors. The guide can help correct these misunderstandings by providing clear examples of how various variables influence outcomes. It offers a detailed breakdown of the typical patterns, helping to identify whether the conditions were truly optimal or if errors may have influenced the data.
For instance, if data suggests no noticeable change over time, referring to the guide might reveal that certain environmental factors were not properly controlled. This can lead to corrective actions for future experiments.
| Expected Outcome | Observed Result | Possible Explanation |
|---|---|---|
| Increase in volume due to solvent movement | No volume change | Improper solution concentration or membrane failure |
| Equilibrium reached between solutions | Imbalance after extended time | Incorrect timing or inconsistent solute concentration |
| Significant change in mass | Minimal change in mass | Environmental temperature fluctuations or measurement error |
By using a reference guide to compare results, the experimenter can identify areas that require attention or clarification, thus ensuring more accurate conclusions in future trials.