
Preparing for a significant academic evaluation requires both understanding key principles and developing effective problem-solving skills. This guide is designed to help you navigate the complex concepts often tested in a scientific assessment, providing strategies to approach each section with confidence.
Thorough preparation is the key to tackling a wide range of topics, from theoretical concepts to practical applications. By focusing on core ideas and practicing various types of problems, you can ensure a comprehensive grasp of the material. A well-rounded approach will enable you to excel under pressure and achieve the best results possible.
In this guide, we will explore different methods to review essential material, offering tips to solve problems efficiently and avoid common pitfalls. With the right tools and mindset, you’ll be ready to approach any task and demonstrate your knowledge effectively.
Chemistry Final Exam Questions and Answers
Preparing for a major assessment in science requires careful review of fundamental topics, as well as honing the skills needed to tackle complex problems. By focusing on key concepts, you can develop a deeper understanding and approach tasks with greater accuracy. A combination of theoretical knowledge and practical exercises is essential for success.
Below, you will find a list of typical problems commonly encountered in such evaluations. Each one is designed to test your grasp of the material and your ability to apply principles in various scenarios. Reviewing these examples can help you identify areas to focus on and improve your problem-solving techniques.
| Topic | Problem Type | Key Skill |
|---|---|---|
| Atomic Structure | Identification of subatomic particles | Understanding atomic composition |
| Periodic Table | Element categorization | Recognizing trends and properties |
| Balancing Reactions | Equation completion | Applying stoichiometric relationships |
| Acids and Bases | pH calculation | Understanding acid-base equilibria |
| Thermodynamics | Energy changes in reactions | Calculating enthalpy changes |
Key Concepts to Review for Exams
Focusing on the core principles that frequently appear in assessments is essential for achieving a strong performance. Understanding fundamental ideas is crucial for tackling a wide variety of tasks. Below are some of the most important topics to prioritize in your review sessions.
- Atomic Structure: Understanding the arrangement of protons, neutrons, and electrons is vital for answering a range of problems.
- Periodic Table Trends: Review the properties and behaviors of elements based on their position on the table, such as electronegativity and ionization energy.
- Chemical Bonding: Be sure to understand covalent, ionic, and metallic bonds, as well as their implications for molecular structure and reactivity.
- Reaction Types: Study the different types of reactions, including synthesis, decomposition, combustion, and redox reactions.
- Stoichiometry: Master the ability to calculate quantities of reactants and products using molar relationships in balanced equations.
- Thermodynamics: Focus on concepts such as energy transfer, enthalpy, entropy, and Gibbs free energy.
- Acid-Base Equilibria: Review the calculation of pH, pOH, and the behavior of acids and bases in aqueous solutions.
- Electrochemistry: Familiarize yourself with redox reactions, electrochemical cells, and standard electrode potentials.
- Gas Laws: Understand the relationships between pressure, volume, temperature, and the amount of gas in various contexts.
Common Types of Chemistry Exam Questions
In any assessment focused on scientific knowledge, certain types of tasks tend to appear more frequently than others. Understanding the format of these problems can help you approach them with greater ease and confidence. The following are some of the most commonly encountered problem types that you will likely face.
Multiple Choice Problems
These tasks test your ability to quickly identify the correct response from a set of options. They often cover a broad range of topics, from basic definitions to more complex concepts. It is important to read each option carefully and eliminate obviously incorrect answers before making a final choice.
Problem-Solving Scenarios
These questions require applying theoretical knowledge to real-world situations. You may be asked to calculate the outcome of a reaction, balance equations, or determine the properties of a substance under different conditions. Focus on clearly understanding the concepts and applying them step by step to avoid mistakes.
Understanding Chemical Reactions in Detail
Mastering the processes involved in transformations of substances is essential for solving many types of problems. Each reaction involves the breaking and forming of bonds, with energy being either released or absorbed. A deep understanding of these processes will allow you to predict the behavior of substances in various conditions and calculate the results accurately.
To truly grasp these changes, it is crucial to familiarize yourself with reaction types, such as synthesis, decomposition, combustion, and displacement reactions. Additionally, understanding how these processes are influenced by factors like temperature, pressure, and concentration will help you analyze and solve related problems effectively.
Tips for Memorizing Chemical Formulas

Memorizing formulas for different substances can be a challenging task, but it is crucial for solving problems effectively. The key to success lies in developing strategies that make recalling these formulas easier and more efficient. Here are some methods that can help you master them.
- Understand the Basics: Focus on the core elements and their charges, such as knowing that sodium (Na) forms a +1 ion and chlorine (Cl) forms a -1 ion.
- Use Mnemonics: Create memorable phrases or associations to help remember complex formulas. For example, “Cows Love Milk” for CaO and Cl2.
- Practice Regularly: Repetition is one of the most effective ways to commit formulas to memory. Write them down, say them aloud, and use flashcards.
- Group Similar Compounds: Group compounds with similar structures together. Recognizing patterns can make it easier to recall specific formulas.
- Use Visual Aids: Drawing diagrams or using models to visualize the structure of compounds can help reinforce memory.
How to Approach Multiple Choice Questions
Multiple-choice tasks often require quick thinking and the ability to narrow down options based on your knowledge. The format tests your ability to identify the correct response from a list of possibilities, making it essential to stay focused and methodical when answering. By applying certain strategies, you can increase your chances of selecting the right choice.
Start by carefully reading the prompt to understand what is being asked. Eliminate any obviously incorrect options first, which will increase your odds of selecting the right one. If you are unsure, try to use logic and recall related concepts that can guide you toward the best answer. If time allows, review your selections to ensure accuracy.
Effective Strategies for Answering Essays
Essay-based tasks often require a deeper level of analysis and the ability to organize thoughts clearly. To perform well, it’s important to approach these types of problems with a structured mindset, ensuring that your response is both comprehensive and focused. By using specific techniques, you can strengthen your ability to present well-reasoned arguments and explanations.
Plan Before Writing
Start by reading the prompt carefully to understand what is being asked. Outline your main points before you begin writing, ensuring that each section of your essay flows logically. This preparation will help you stay on topic and avoid unnecessary tangents.
Support Your Arguments
When presenting your points, always back them up with relevant examples, explanations, or calculations. Strong evidence makes your argument more convincing and shows a thorough understanding of the material. Be sure to also address potential counterarguments if applicable, as this demonstrates critical thinking and depth of knowledge.
Practice Problems for Better Understanding
Regularly working through problems is one of the most effective ways to solidify your knowledge and improve your problem-solving abilities. By practicing different types of exercises, you reinforce key concepts and develop the skills needed to approach similar challenges confidently. The more you practice, the more intuitive the material becomes.
Focusing on a variety of tasks, ranging from simple calculations to more complex scenarios, allows you to apply theoretical knowledge in different contexts. Whether through textbook exercises, online resources, or practice sets, consistent practice helps identify areas for improvement and builds a strong foundation for tackling related problems in assessments.
How to Tackle Organic Chemistry Questions
Organic tasks often involve understanding the structure, behavior, and reactions of carbon-based compounds. These problems can range from identifying functional groups to predicting reaction outcomes. Developing a systematic approach will allow you to navigate these tasks with confidence and precision.
Master the Basics
Begin by ensuring that you have a solid understanding of basic concepts such as bonding, functional groups, and nomenclature. These fundamental principles form the foundation for solving more complex challenges. Having this knowledge at your fingertips allows you to identify key features of molecules and predict their reactions effectively.
Practice Reaction Mechanisms
Focus on understanding the step-by-step processes involved in organic reactions. Memorize common reaction mechanisms and be able to visualize how electrons move during each step. This approach will help you anticipate the products of a reaction and avoid common mistakes when solving related tasks.
Reviewing Periodic Table Trends
Understanding the patterns that govern the properties of elements can significantly enhance your ability to predict their behavior. The arrangement of elements in the periodic chart reveals trends that reflect their atomic structure and chemical reactivity. By reviewing these patterns, you can gain deeper insights into elemental properties and how they interact in various situations.
Trends in Atomic Size
The atomic radius tends to increase as you move down a group due to the addition of electron shells. Conversely, as you move across a period from left to right, the radius decreases. This happens because the number of protons in the nucleus increases, pulling the electrons closer to the center.
Electronegativity and Ionization Energy
Electronegativity is a measure of an atom’s ability to attract electrons in a bond. It increases across a period and decreases down a group. Similarly, ionization energy, which is the energy required to remove an electron from an atom, increases across periods and decreases down groups, reflecting how tightly an atom holds onto its electrons.
Balancing Chemical Equations Made Easy
Balancing reactions is a fundamental skill in solving many types of problems. A balanced equation ensures that the number of atoms of each element is the same on both sides of the equation, reflecting the law of conservation of mass. By following a few simple steps, you can easily balance any reaction.
Steps to Balance Equations
- Write the unbalanced equation: Start by writing the reactants and products as they are given in the problem.
- Count atoms: Count the number of atoms of each element on both sides of the equation.
- Adjust coefficients: Begin adjusting the coefficients (the numbers in front of molecules) to balance the atoms. Start with the most complex molecule.
- Balance one element at a time: Focus on balancing one element at a time, leaving hydrogen and oxygen for last.
- Check your work: After balancing, count the atoms again to ensure everything matches on both sides of the equation.
Common Mistakes to Avoid
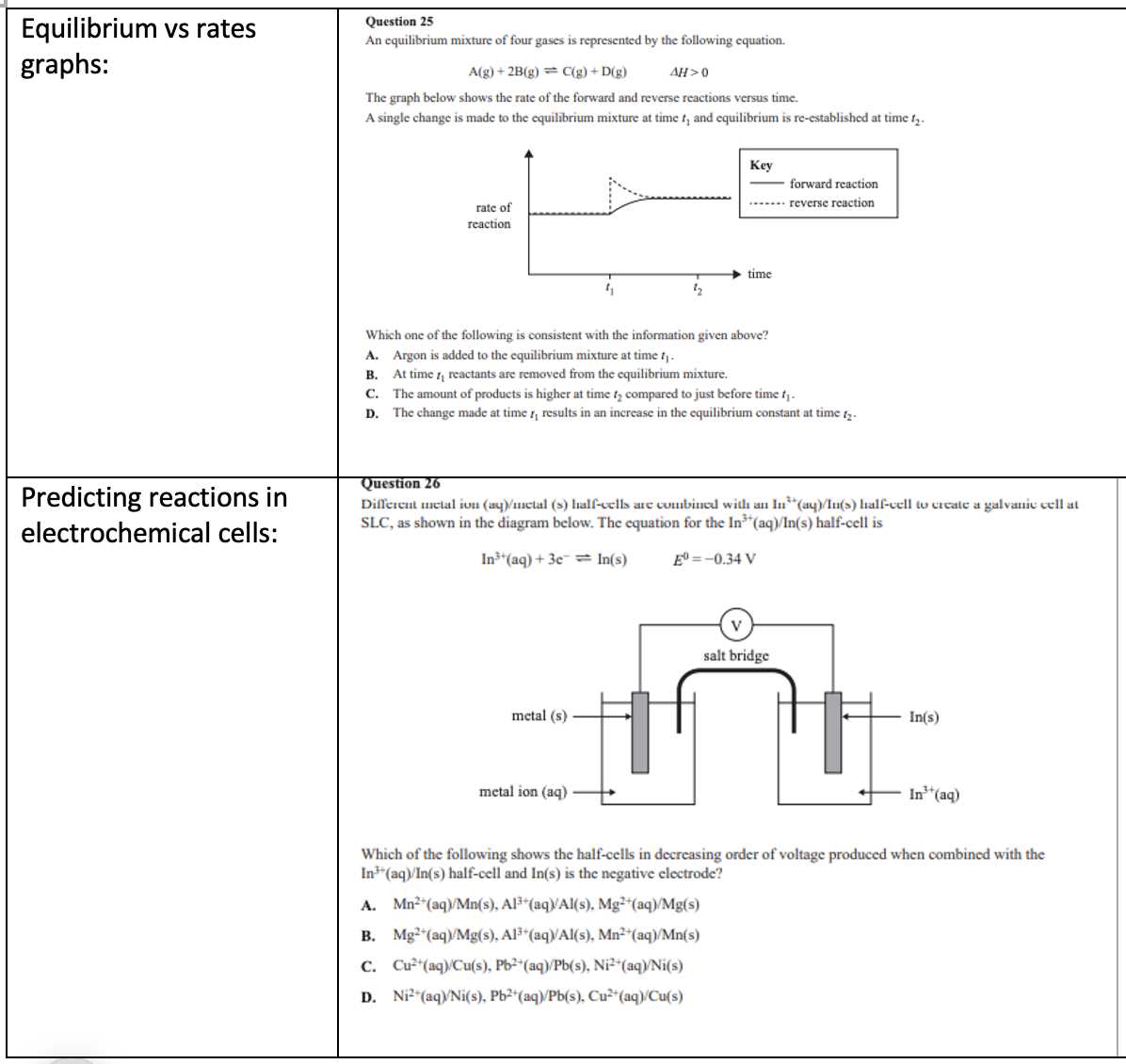
- Skipping coefficients: Never change the subscripts (the small numbers inside the formulas), only adjust the coefficients.
- Leaving atoms unbalanced: Ensure that each element is balanced before finalizing the equation.
- Rushing the process: Take your time to carefully check your work, especially if the reaction involves multiple elements.
Preparing for Stoichiometry Questions
Mastering stoichiometry is crucial for solving problems that involve the quantitative relationships between reactants and products in a chemical reaction. This topic requires a solid understanding of molar ratios, unit conversions, and the ability to apply dimensional analysis effectively. Preparing for these types of challenges involves not just memorization but also developing a logical approach to problem-solving.
To excel in these problems, begin by reviewing the steps involved in converting from one substance to another, using conversion factors like moles, grams, and molecules. Being comfortable with the periodic table and understanding how to use it for finding molar masses will streamline the process. Practicing with sample exercises is key to gaining confidence and improving your speed in identifying the correct path for solving each problem.
How to Solve Thermodynamics Problems
Solving problems related to energy changes and heat transfer involves understanding fundamental concepts such as enthalpy, entropy, and the laws that govern energy in systems. These problems require a clear approach to applying formulas and concepts to determine the outcome of processes. Mastering thermodynamics involves breaking down the problem into manageable steps and focusing on key principles like energy conservation and system efficiency.
Steps to Approach Thermodynamic Problems
- Identify the system: Determine what is being measured or observed, whether it’s heat flow, work done, or energy change.
- Understand the laws: Familiarize yourself with the first and second laws of thermodynamics, and how they apply to the problem.
- Set up equations: Use the appropriate formulas for heat (Q), work (W), or internal energy changes (ΔU). Ensure you understand which variables are given and which need to be calculated.
- Use stoichiometry: When dealing with reactions, convert quantities between moles and grams, using stoichiometric relationships to find the required values.
- Check units: Ensure all units are consistent before performing calculations to avoid errors in your final answer.
Common Pitfalls to Avoid

- Ignoring the sign convention: Pay attention to whether energy is absorbed or released (positive or negative values) in your calculations.
- Overlooking equilibrium: In certain problems, equilibrium states need to be factored in, especially when calculating changes in entropy or Gibbs free energy.
- Forgetting to convert units: Always convert temperature, pressure, or volume to the correct units before solving.
Importance of Lab-Based Questions
Practical assessments play a crucial role in understanding the application of theoretical concepts in real-world scenarios. These types of tasks allow individuals to demonstrate their ability to apply learned knowledge to hands-on situations, fostering a deeper understanding of the material. Engaging with laboratory-related tasks tests not only theoretical comprehension but also the ability to conduct experiments, analyze results, and draw conclusions.
Such challenges often involve interpreting data, using equipment correctly, and ensuring safety protocols are followed. Mastering these aspects can significantly enhance one’s problem-solving skills and critical thinking, which are essential for academic success and practical experience. Preparing for these tasks involves more than memorizing formulas; it requires practice in thinking analytically and troubleshooting in real-time under controlled conditions.
Time Management During the Exam
Effective use of time is crucial for success in any assessment setting. Being able to allocate appropriate amounts of time to different sections can help maximize performance and reduce stress. Good time management allows individuals to carefully consider each task, avoid rushing, and ensure that all aspects of the test are completed to the best of their ability.
It is important to have a strategy in place before beginning the test. This can include quickly scanning the entire paper to get a sense of the sections that require more time and those that can be completed more quickly. Prioritizing tasks, staying aware of the time left, and pacing oneself appropriately are key elements in ensuring that no section is overlooked. Staying calm and maintaining focus also helps to maintain efficiency throughout the process.
Common Mistakes to Avoid in Chemistry Exams
Understanding the key concepts is essential, but it’s just as important to approach the assessment methodically to avoid simple errors that can cost valuable marks. Many students fall into common traps due to haste, lack of attention to detail, or misunderstanding the task at hand. By being aware of these pitfalls, individuals can refine their approach and improve their chances of success.
1. Misinterpreting Instructions

One of the most common mistakes is misreading the instructions. Whether it’s the format of the answer or specific guidelines for a problem, misunderstanding the task can lead to incorrect responses. Always take a moment to carefully read each question and ensure you know what is being asked.
2. Overlooking Unit Conversions

Unit conversions are often overlooked, leading to incorrect calculations or incomplete answers. Always double-check that the correct units are used, and ensure any necessary conversions are made before finalizing answers.
| Mistake | Why It Happens | How to Avoid It |
|---|---|---|
| Skipping Steps in Calculations | Rushing through problems | Write down all steps clearly to avoid missing anything |
| Forgetting to Label Answers | Overlooking small details | Always check that answers are complete with correct labels |
| Incorrectly Balancing Equations | Lack of practice | Review balancing techniques regularly to build confidence |
By paying close attention to these details and preparing effectively, it’s possible to avoid these common errors and perform more accurately under pressure.