
In the study of chemical reactions, the concept of equilibrium plays a crucial role in understanding how reactions proceed under various conditions. The balance between forward and reverse processes is essential for predicting the behavior of substances in a reaction mixture. Whether reactions shift toward more products or reactants, the ability to quantify this shift is fundamental for chemists in both theoretical and practical applications.
The mathematical framework behind these shifts involves calculating specific ratios that describe the current state of a reaction. These ratios help to assess whether a system is at equilibrium or if changes are occurring in the reaction direction. By analyzing these values, scientists can predict how altering conditions like concentration or temperature might influence the system.
Through various learning approaches, students can gain a deeper understanding of these critical concepts. Practical exercises allow learners to apply theoretical knowledge to real-world scenarios, strengthening their grasp of how chemical processes are balanced. Mastery of this material forms the foundation for more complex studies in chemistry and its applications in different fields.
Understanding the Key Concept of Chemical Balance
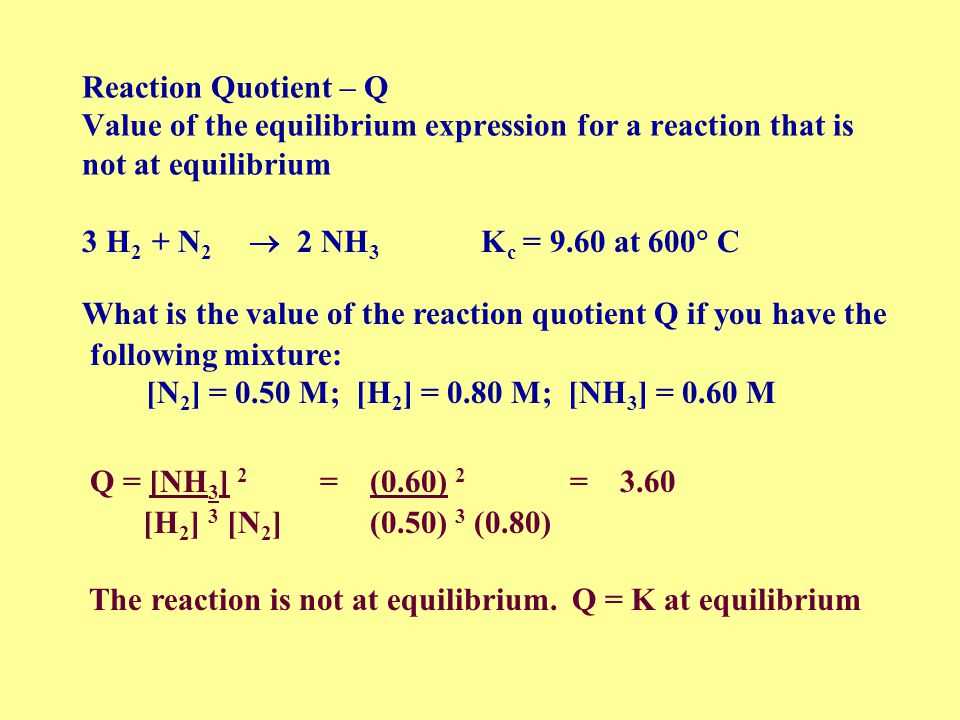
In chemical reactions, understanding how a system behaves during the process of change is essential. Reactions do not always proceed until all reactants are consumed; instead, they often reach a point where the concentrations of products and reactants remain constant over time. This balance between the two sides of a reaction can be quantified and analyzed to provide insights into the direction and extent of the reaction.
At this stage, the ratio of product and reactant concentrations plays a significant role in understanding the dynamics of the system. By comparing these values at any given moment, it becomes possible to determine whether the system is shifting toward more products, more reactants, or remaining in a steady state. This ratio, calculated under specific conditions, offers valuable information about the system’s equilibrium status and how it responds to changes in the environment, such as pressure, temperature, or concentration.
Applying these principles allows chemists to predict how reactions will behave under various circumstances. By gaining a deeper understanding of these ratios, students can better grasp the subtleties of chemical equilibrium and its real-world implications in fields such as pharmaceuticals, environmental science, and industrial chemistry.
What is the Key Ratio in Chemistry?
In chemical processes, the relationship between the amounts of reactants and products at any given moment provides crucial information about the state of the reaction. This ratio is used to assess whether the system has reached a stable condition or if it is still moving toward equilibrium. The concept allows chemists to predict the behavior of reactions under various conditions and make informed decisions about how to control or modify these processes.
Defining the Key Ratio
This ratio is derived by comparing the concentrations of products to those of reactants. The result provides insight into whether the reaction is moving forward or backward, or if the system is at rest. A change in this ratio indicates a shift in the reaction’s progress, helping chemists identify whether additional measures, such as adjusting temperature or pressure, are required to maintain or change the system’s behavior.
How It Works in Practice
In practice, this concept is used to analyze real-time changes in chemical systems. Some important points to consider include:
- The ratio reflects the dynamic nature of chemical equilibrium, where both forward and reverse reactions occur at the same rate.
- A ratio greater than 1 suggests that the products are more abundant, while a ratio less than 1 indicates a higher concentration of reactants.
- Changes in external factors, such as temperature or pressure, can affect this ratio and shift the system’s balance.
By understanding this fundamental concept, students and professionals alike can gain a deeper appreciation for how chemical systems behave and how to manipulate them for various applications, including industrial processes and laboratory experiments.
POGIL Approach to Understanding Chemical Ratios
The Process-Oriented Guided Inquiry Learning (POGIL) approach offers a student-centered method to mastering complex concepts in chemistry. By focusing on active learning and inquiry-based activities, this approach encourages students to explore key principles through guided discovery rather than passive reception of information. In the context of chemical systems, POGIL helps learners understand the relationship between products and reactants by promoting problem-solving and critical thinking.
Key Benefits of the POGIL Method
The POGIL approach fosters a deeper understanding by breaking down the problem into manageable steps. Instead of simply memorizing formulas, students engage with real-world examples and experiments, allowing them to observe how various factors influence the balance between substances. The interactive nature of this method helps reinforce concepts by requiring students to apply their knowledge actively.
Structure of a POGIL Activity
Each POGIL activity is structured to guide students through a sequence of questions that build upon one another, encouraging independent thought and collaboration. Below is an example structure for a typical activity on chemical ratios:
| Step | Description |
|---|---|
| 1 | Introduction to the Problem: Students are presented with a scenario involving a chemical system. |
| 2 | Exploration: Learners work through questions that help identify key relationships between substances. |
| 3 | Concept Application: Students apply what they have learned to solve more complex problems or case studies. |
| 4 | Reflection: Discussion and review of the concepts to solidify understanding and address any misconceptions. |
Through this structured, step-by-step process, students gain a solid understanding of how the concentration ratios between reactants and products evolve throughout a reaction. This method not only clarifies theoretical concepts but also helps learners develop essential problem-solving skills.
Key Differences Between Q and K
In chemical processes, two important ratios are used to describe the concentrations of products and reactants: one that applies at any moment in time and another that represents equilibrium conditions. Although these two ratios are closely related, they serve different purposes and are used in distinct contexts. Understanding the differences between them is essential for predicting the direction of a reaction and determining if the system is in a state of equilibrium.
Fundamental Distinctions
Here are the primary differences between these two key values:
- Purpose: One ratio helps assess the current state of a reaction, while the other describes the balance when the system has reached equilibrium.
- Calculation Time: The first ratio is calculated at any point during the reaction, whereas the second is specifically calculated when no further changes in concentration occur.
- Reaction Progress: The first ratio can be used to determine whether the system is moving towards equilibrium or if it has already stabilized. The second ratio is a constant for a given reaction under specific conditions.
When to Use Each Ratio
The use of each ratio depends on the stage of the chemical process:
- During a reaction: The first ratio is more useful when trying to determine how far along the reaction is and in which direction it is progressing.
- At equilibrium: The second ratio is used to evaluate whether the reaction has reached a stable state, with no net changes in the concentrations of products and reactants.
By understanding these key differences, chemists can better predict how changes in external factors, such as temperature or pressure, will affect the system and whether additional adjustments are needed to reach or maintain equilibrium.
Step-by-Step Guide to Calculating Q
To understand the progression of a chemical process, it is essential to calculate the ratio of product to reactant concentrations at any given point in time. This calculation allows chemists to assess whether the system is in equilibrium or shifting toward it. By following a systematic approach, one can accurately determine the status of the system and make predictions about its future behavior.
Step 1: Identify the Balanced Equation
The first step in calculating the ratio is to have a balanced equation for the chemical system. This equation tells you the stoichiometric relationship between the substances involved. It’s essential to ensure that the number of atoms for each element is the same on both sides of the equation.
Step 2: Gather Concentration Data

Next, collect the concentration values of the products and reactants. These values can either be provided in the problem statement or measured experimentally. If any concentrations are missing, you may need to calculate them based on the reaction conditions or other known data.
Step 3: Write the Ratio Expression
Using the concentrations, write the expression for the ratio. The general formula involves placing the concentrations of products in the numerator and the concentrations of reactants in the denominator, each raised to the power of their respective coefficients in the balanced equation.
Example: For the reaction aA + bB ⇌ cC + dD, the ratio would be written as:
Q = [C]^c [D]^d / [A]^a [B]^b
Step 4: Plug in the Values
Substitute the concentration values into the ratio expression. Ensure that the units are consistent across all substances, and use the correct stoichiometric coefficients for each species involved in the reaction.
Step 5: Solve the Expression
After inserting the concentration values, solve the expression. This will give you the value of the ratio at that moment in time. If the value is greater than 1, it indicates that the products are favored; if less than 1, the reactants are favored.
By following these steps, you can calculate the ratio at any point during a chemical process and gain valuable insight into the direction of the reaction. This calculation is crucial for understanding and manipulating chemical systems in both academic and real-world applications.
How to Apply Key Ratios in Reactions
Understanding how to apply key ratios during a chemical process is essential for determining the direction of a reaction and whether it is at equilibrium. By calculating this ratio at any given moment, scientists can predict how the system will behave under different conditions, such as changes in temperature, pressure, or concentration. This method allows for a better understanding of how to manipulate reaction conditions to achieve desired outcomes.
Practical Applications of the Ratio
The ratio provides valuable information about the current state of a reaction. Here are the key ways it can be applied:
- Determining Reaction Progress: When the ratio is calculated, comparing it to the equilibrium constant allows chemists to understand whether the system is moving toward more products or reactants.
- Predicting Shifts: A comparison of the ratio with the equilibrium constant can indicate whether external changes, such as adding or removing substances, will shift the reaction towards the products or the reactants.
- Evaluating System Behavior: By tracking the ratio over time, you can determine if the system is approaching equilibrium or if more adjustments are needed to reach it.
Steps to Apply the Key Ratio
To apply this ratio in a real-world scenario, follow these steps:
| Step | Action |
|---|---|
| 1 | Write the balanced equation for the system. |
| 2 | Gather the concentration values of the substances involved in the reaction. |
| 3 | Substitute the concentrations into the ratio expression. |
| 4 | Compare the calculated ratio with the equilibrium constant. |
| 5 | Predict the direction in which the reaction will shift. |
By applying this method systematically, you can predict how changes in concentration, temperature, or pressure will influence the reaction, allowing for precise control over the chemical process.
Common Mistakes in Chemical Ratio Problems
When solving problems involving the ratio of product to reactant concentrations, it’s easy to make mistakes that can lead to incorrect conclusions. These errors can arise from simple misunderstandings of the process, incorrect data handling, or improper application of principles. Recognizing and addressing these common pitfalls will help ensure accurate results and a better understanding of the system being studied.
Frequent Errors in Calculations
Here are some of the most common mistakes students and practitioners make when working with these types of problems:
- Incorrectly Writing the Expression: A frequent error is incorrectly writing the formula for the ratio. Be sure to correctly place the concentrations of products in the numerator and reactants in the denominator, raised to the power of their coefficients in the balanced equation.
- Forgetting to Include All Substances: Sometimes, students forget to include all the substances involved in the reaction, especially if some have a concentration of zero. Even though zero concentration substances do not contribute to the ratio, they must still be accounted for in the equation.
- Using Incorrect Units: Failing to use consistent units for concentration can lead to confusion or incorrect results. Always ensure that concentration values are in the same units throughout the calculation.
- Confusing Equilibrium with Non-Equilibrium: It’s important to differentiate between the equilibrium ratio and the non-equilibrium ratio. The two have different applications and should not be confused, as this can lead to the wrong predictions regarding the direction of the reaction.
- Ignoring Stoichiometric Coefficients: Another common mistake is forgetting to apply the correct stoichiometric coefficients when raising the concentrations to their respective powers. This will lead to inaccurate calculations and misinterpretation of the system.
How to Avoid These Mistakes
To avoid these common pitfalls, follow these tips:
- Carefully Review the Balanced Equation: Always double-check the stoichiometric coefficients and make sure all substances are accounted for in the expression.
- Verify Units: Ensure all concentration units are consistent before starting the calculation.
- Check if the System is at Equilibrium: Make sure you understand whether the problem involves equilibrium or non-equilibrium conditions, as this will affect how you use the ratio.
- Practice Problem-Solving: The more you practice, the more familiar you will become with the typical mistakes and how to avoid them.
By understanding and avoiding these common errors, you can improve your accuracy and confidence in solving problems related to chemical systems.
Why the Key Ratio Matters
The key ratio is a fundamental tool in chemistry, offering insight into the state of a system during a chemical process. It helps determine whether a reaction is in equilibrium or moving toward it, providing essential information on how a system behaves over time. This knowledge allows scientists to manipulate conditions such as concentration, temperature, or pressure to drive reactions in the desired direction.
Understanding Its Importance
There are several reasons why this ratio is so crucial in chemical studies:
- Indicating Direction of Reaction: By comparing the ratio at any given moment to the equilibrium constant, you can predict the direction in which the system will move–either towards products or reactants.
- Monitoring Progress: Tracking this ratio over time can show how far the reaction has progressed and whether equilibrium has been reached.
- Optimizing Reaction Conditions: Understanding the ratio allows for better control of the system, enabling adjustments to concentration or temperature that may accelerate the reaction or improve yields.
- Predicting Shifts: If the system is not at equilibrium, this ratio can guide predictions about how changes in concentration or pressure will influence the equilibrium state.
How It Supports Chemical Engineering
In industrial applications, the ability to control chemical reactions is essential for efficiency and safety. The key ratio plays a central role in scaling reactions for production. By monitoring and adjusting this ratio, chemists and engineers can ensure that reactions are proceeding as efficiently as possible, reducing waste and increasing product yield.
For example:
| Scenario | Action | Outcome |
|---|---|---|
| High Product Concentration | Shift the system to produce more reactants. | Increased product yield when the reaction is pushed toward equilibrium. |
| Low Reactant Concentration | Introduce more reactants to shift the reaction. | Reaction favors the formation of more products. |
Ultimately, understanding and applying this ratio allows chemists and engineers to make better decisions, optimize processes, and improve overall efficiency in both laboratory and industrial settings.
Equilibrium vs Non-Equilibrium Conditions Explained
In any chemical system, understanding the difference between equilibrium and non-equilibrium conditions is essential for predicting how the system will behave. These two states dictate how the substances involved in the process are distributed, and how they will respond to changes in factors like concentration, pressure, or temperature. This distinction is crucial for controlling and manipulating chemical processes effectively.
Key Differences Between the Two Conditions
At equilibrium, a system reaches a point where the concentrations of products and reactants remain constant over time, as the forward and reverse processes occur at the same rate. In contrast, non-equilibrium conditions occur when the system has not yet reached this state, and there is still a net change in the concentrations of substances.
| Condition | Equilibrium | Non-Equilibrium |
|---|---|---|
| Concentrations of substances | Constant over time | Changing over time |
| Rate of forward and reverse reactions | Equal rates | Unequal rates |
| System behavior | No net change in concentrations | Ongoing changes in concentrations |
| External conditions | Stable and balanced | Shifting due to imbalances |
Why the Distinction Matters
Understanding whether a system is in equilibrium or non-equilibrium helps determine how the reaction will proceed and what steps need to be taken to influence it. In equilibrium, the system has reached a stable state, and no further changes will occur unless external factors are applied. In non-equilibrium conditions, the reaction is still progressing, and adjustments can be made to influence the direction and speed of the process.
For instance, in industrial processes, it is crucial to monitor whether a system is at equilibrium to avoid wasting resources or energy. In non-equilibrium systems, however, engineers can optimize the conditions to drive the reaction toward the desired product more efficiently.
Analyzing Data with Key Chemical Ratio
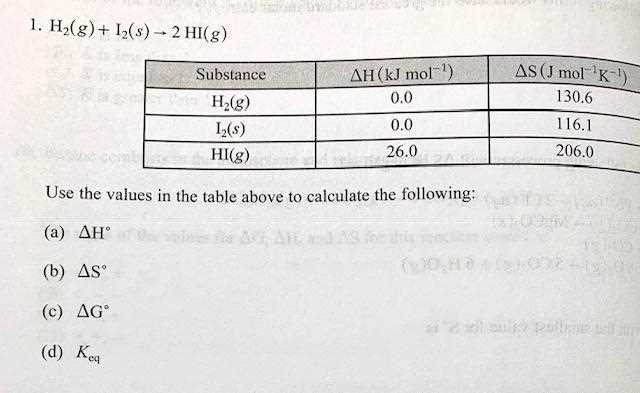
When studying chemical systems, analyzing data through the key chemical ratio provides valuable insight into the progress and direction of a process. This ratio offers a snapshot of the relationship between products and reactants at a given moment, allowing chemists to assess whether a system is at equilibrium or if changes are necessary to achieve balance. Proper analysis of this data can guide decisions on how to manipulate conditions to achieve the desired outcome.
Steps to Analyze the Data
To analyze data effectively, it is important to follow a few systematic steps:
- Collect Relevant Data: Gather concentration values for both products and reactants at various points during the process.
- Calculate the Key Ratio: Use the concentrations of substances involved to compute the ratio at the given moment.
- Compare with Equilibrium Constant: Assess whether the ratio is greater than, less than, or equal to the equilibrium constant (K). This comparison helps determine if the system is in equilibrium or which direction the reaction needs to proceed in.
- Adjust Conditions as Needed: Based on the analysis, make adjustments to the system’s conditions, such as concentration, temperature, or pressure, to achieve the desired balance.
Interpreting the Data
Once the key ratio is calculated, interpreting the results is essential for understanding the state of the system:
- If the ratio is less than the equilibrium constant (K), the reaction is likely moving toward the formation of more products.
- If the ratio is greater than K, the system will tend to shift towards producing more reactants.
- If the ratio is equal to K, the system is at equilibrium, and no further changes will occur unless external factors are applied.
Through careful analysis of this ratio and the surrounding data, chemists can make informed decisions about how to manipulate and optimize chemical reactions for various applications, from laboratory experiments to industrial processes.
Reaction Quotient in Dynamic Systems
In dynamic chemical systems, the relationship between products and reactants continually changes until equilibrium is reached. These systems are constantly evolving, with molecules interacting and shifting back and forth between different states. The ability to monitor and understand these shifts is crucial for predicting the future behavior of the system. The ratio that represents the balance between substances at any given moment can provide essential insights into how the system is progressing toward equilibrium.
In a dynamic system, concentrations of reactants and products are not static. As the reaction progresses, their concentrations fluctuate, and the system continuously adjusts to external conditions. By measuring and analyzing the key ratio at different points, we can determine the current state of the system and predict its eventual equilibrium state. This dynamic nature allows chemists to control and influence chemical reactions by modifying the conditions, such as temperature or concentration, to push the system toward a desired outcome.
In such systems, the key ratio serves as a tool for understanding the direction of change and the rate at which it occurs. It helps identify whether the system is moving toward producing more products or converting back to reactants, thus guiding interventions for optimal results. This dynamic monitoring is essential for fields ranging from industrial chemistry to environmental science, where maintaining or shifting equilibrium states is often necessary for desired reactions and processes.
Real-Life Examples of Chemical Ratios
The application of chemical ratios in real-life scenarios helps us understand and manipulate various processes in nature and industry. These ratios provide insight into how a chemical system behaves at any given moment and whether it is moving toward a state of equilibrium. From industrial manufacturing to biological systems, understanding how these ratios function can be crucial for optimizing processes and achieving desired outcomes.
1. Industrial Manufacturing Processes
In industries such as chemical production, the ability to control the balance of products and reactants is essential for maximizing efficiency and minimizing waste. One common example is the Haber process for synthesizing ammonia, where controlling the ratio of nitrogen and hydrogen gases determines the yield of ammonia. By monitoring the key ratio of reactants and products, engineers can adjust temperature, pressure, or other variables to push the system toward producing more ammonia efficiently.
2. Biological Systems
Biological systems also rely on dynamic chemical balances. For example, the human body maintains a delicate balance of gases like oxygen and carbon dioxide in the bloodstream. When cells undergo metabolism, these gases are exchanged, and the body monitors their concentrations to ensure proper functioning. Changes in this balance can lead to conditions like acidosis or alkalosis, which can affect cellular function. By studying the ratios in such systems, healthcare professionals can better understand and treat metabolic disorders.
By analyzing these examples, we see how crucial it is to understand the role of chemical ratios in both industrial applications and biological processes. With this knowledge, it is possible to fine-tune conditions to optimize outcomes, whether it be in a manufacturing plant or within living organisms.
Reaction Quotient and Chemical Equilibrium
In any chemical process, the system evolves toward a state where the rate of conversion of reactants to products equals the rate of conversion of products back to reactants. This balance, known as equilibrium, is central to understanding how reactions proceed and how they can be influenced. The measurement of the relative concentrations of reactants and products at any given point in the process provides valuable information about where the system stands in relation to equilibrium.
By examining the key ratio at various stages, it becomes possible to predict the direction in which the system is shifting. If the ratio is greater than the equilibrium value, the system will shift toward producing more reactants. Conversely, if the ratio is less than the equilibrium value, the system will shift toward generating more products. This concept allows chemists to manipulate conditions–such as pressure, temperature, or concentration–to drive reactions toward a desired outcome, whether it’s the production of more products or the preservation of reactants.
Understanding this relationship between concentrations and equilibrium helps in diverse fields, from industrial chemistry, where maximizing product yield is essential, to environmental science, where controlling pollutant levels is critical. By continually monitoring and adjusting the system, one can ensure it operates optimally and sustainably.
How Temperature Affects Chemical Balance
Temperature plays a crucial role in determining the direction and rate of chemical reactions. As the temperature of a system changes, the energy of the molecules involved in the process also changes, which can affect how reactants and products interact. This shift in energy impacts the equilibrium state, influencing the concentration of reactants and products at any given moment.
When the temperature is increased, the system may favor either the forward or reverse reaction depending on the nature of the process. For exothermic reactions, where energy is released, raising the temperature generally shifts the equilibrium toward the reactants, as the system tries to counteract the added heat. Conversely, for endothermic reactions, where energy is absorbed, increasing the temperature often shifts the equilibrium toward the products. This effect is described by Le Chatelier’s Principle, which states that the system will adjust to minimize the impact of changes in temperature.
Understanding how temperature influences chemical balance is essential in industries such as chemical manufacturing, where controlling reaction conditions is key to maximizing yield and minimizing energy consumption. By carefully adjusting temperature, chemists can optimize reaction rates and control the direction of the process, ensuring efficient production and desired outcomes.
Predicting Reaction Direction with Q
Understanding how a system will evolve is a critical aspect of chemical analysis. By examining the ratio of concentrations of reactants and products at any given moment, it is possible to predict whether a reaction will proceed forward, reverse, or remain at equilibrium. This approach allows chemists to anticipate how the system will adjust to reach a balanced state, based on the current conditions and concentrations.
When the system is not at equilibrium, the calculated ratio of products to reactants–referred to here as the “current ratio”–can be compared to the value at equilibrium. Based on this comparison, the direction of the reaction can be predicted:
- If the current ratio is smaller than the equilibrium value: The reaction will proceed forward, producing more products to reach equilibrium.
- If the current ratio is larger than the equilibrium value: The reaction will shift in reverse, generating more reactants to restore balance.
- If the current ratio equals the equilibrium value: The system is at equilibrium, and no further changes will occur unless external conditions change.
By applying this concept, chemists can make informed decisions to influence the course of a reaction, adjusting factors such as concentration, temperature, or pressure to optimize the process and achieve desired results efficiently.
POGIL Answering Strategies for Reaction Quotient
When engaging with problems involving the measurement of reaction progress, a structured approach to understanding and solving the related questions is key. By focusing on logical problem-solving steps and effective teamwork, students can improve their ability to tackle complex scenarios. The goal is to break down the problem into manageable parts, ensuring clarity at every stage of the process.
One effective strategy involves first analyzing the problem’s context, focusing on the information given and identifying what is being asked. This helps in setting the stage for understanding how various components of the system interact. Next, students should organize the data into a format that makes comparison and calculations straightforward.
Additionally, collaborative learning plays a pivotal role. Discussing potential answers and reasoning with peers can highlight different perspectives and potential solutions that one might have missed. The process encourages deep understanding by fostering group discussions, which often lead to a clearer approach to solving individual questions.
- Step 1: Identify the components of the system (reactants and products) and their concentrations.
- Step 2: Determine the values needed to calculate the ratio of products to reactants.
- Step 3: Compare the result with equilibrium values to predict the direction of the process.
- Step 4: Discuss results with your peers to confirm your findings and refine the approach.
By following these strategies, students can approach these types of problems systematically, making it easier to find solutions and understand the underlying principles behind chemical changes in dynamic systems.