
In the world of chemical experiments, understanding the energy changes during reactions is essential for accurate results. This concept plays a critical role in predicting the outcome of various processes and ensures that scientific experiments are grounded in precise energy measurements. Knowing how energy flows, whether absorbed or released, can significantly impact the way experiments are designed and interpreted.
Energy transformations are a central theme in this area of study. By examining how heat moves during different transformations, we gain insights into the fundamental behavior of substances under varying conditions. This understanding not only helps in predicting the final state of materials but also guides researchers in optimizing processes for practical applications.
Calculating energy shifts involves identifying the heat transfer at different stages. Various principles help in simplifying these calculations, and mastering them is crucial for anyone looking to excel in scientific experimentation. With the right approach, even complex transformations can be understood in terms of measurable energy changes, offering clear pathways to analysis and discovery.
Thermodynamics and Enthalpy of Reaction

The study of energy changes during chemical processes is vital for understanding how substances interact under various conditions. Every transformation, whether it’s the breakdown of a compound or the formation of new ones, involves a shift in energy. Grasping these energy transitions allows scientists to predict the outcomes of chemical interactions, optimize processes, and explain the behavior of materials when exposed to different environments.
At the heart of this area of study lies the concept of heat transfer. As reactions unfold, heat can either be absorbed from or released into the surroundings, which affects the overall system. This process is essential for determining the conditions required for a specific transformation and understanding the energy flow within a system. Accurate measurement and analysis of this energy shift are key to conducting successful experiments.
The ability to quantify energy changes helps in calculating the potential outcomes of chemical events. This measurement provides a deeper insight into how substances behave at a molecular level, shedding light on the mechanisms that drive their interactions. By using specific principles, scientists can predict how much energy is involved in a given change, guiding them to more efficient and controlled experimental conditions.
Understanding the Basics of Thermodynamics
The foundation of any chemical or physical process lies in the principles that govern energy exchanges. At the core of this study is the understanding of how energy is transferred between systems and their surroundings. This knowledge is essential for predicting how substances behave when they undergo different changes, whether they are breaking down or forming new compounds.
Energy in its various forms, such as heat and work, plays a critical role in these transformations. It’s important to grasp the key concepts related to how energy flows, as this affects the way chemical processes are approached and controlled in experiments.
- System and Surroundings: The system refers to the substances involved in a transformation, while the surroundings are everything outside of it. Understanding the interaction between both helps in analyzing how energy is exchanged.
- Energy Conservation: The principle of conservation ensures that energy is neither created nor destroyed but can only be transferred or converted between different forms.
- Work and Heat: These are the primary methods through which energy is exchanged. Work refers to energy transfer due to a force, while heat is energy transferred due to temperature differences.
Understanding these fundamental concepts allows for more accurate predictions and calculations in any experiment or process involving energy changes. It provides the necessary groundwork for more advanced topics, where these principles are applied to real-world scenarios. Grasping how energy is distributed and conserved during transformations is a crucial first step in studying chemical processes at a deeper level.
Defining Enthalpy in Chemical Reactions
In the context of chemical processes, energy plays a pivotal role in determining how substances interact and change. When a material undergoes a transformation, it either absorbs or releases energy, which can significantly influence the behavior of the system. Understanding how this energy is quantified allows scientists to predict the direction and extent of these changes.
One way to describe this energy exchange is by focusing on the total heat content of the system. This quantity not only reflects the energy absorbed or released but also takes into account the pressure and volume conditions under which the transformation occurs. The change in this value during a process can indicate whether energy is absorbed from the surroundings or released into them, providing a clear picture of the process’s energy dynamics.
To quantify these energy shifts, specific methods are used that account for the internal energy, work, and heat involved. These measurements are crucial for accurately describing chemical events, enabling researchers to predict and control the conditions necessary for desired outcomes. This concept is fundamental to understanding how energy influences the stability and properties of substances in various processes.
Role of Heat in Chemical Processes
Heat is a fundamental factor that influences how substances behave when undergoing various changes. When materials transform, whether by breaking apart or forming new compounds, heat plays a critical role in determining the direction and speed of the process. The amount of heat absorbed or released can directly affect the properties of the substances involved, making it essential to understand its role in these transformations.
During a process, heat can be transferred between the system and its surroundings, either increasing or decreasing the energy within the system. This energy shift can cause changes in temperature, pressure, and volume, which in turn affect how molecules interact. For example, heat can provide the necessary energy for a substance to reach a higher energy state, or it can cause the system to release energy as it moves to a more stable state.
The study of heat in chemical processes allows scientists to predict how materials will behave under different conditions. By measuring heat changes, researchers can gain insights into the efficiency, speed, and stability of these processes. This understanding is key to optimizing conditions for chemical production, controlling reaction rates, and ensuring the desired outcomes in various industrial and laboratory applications.
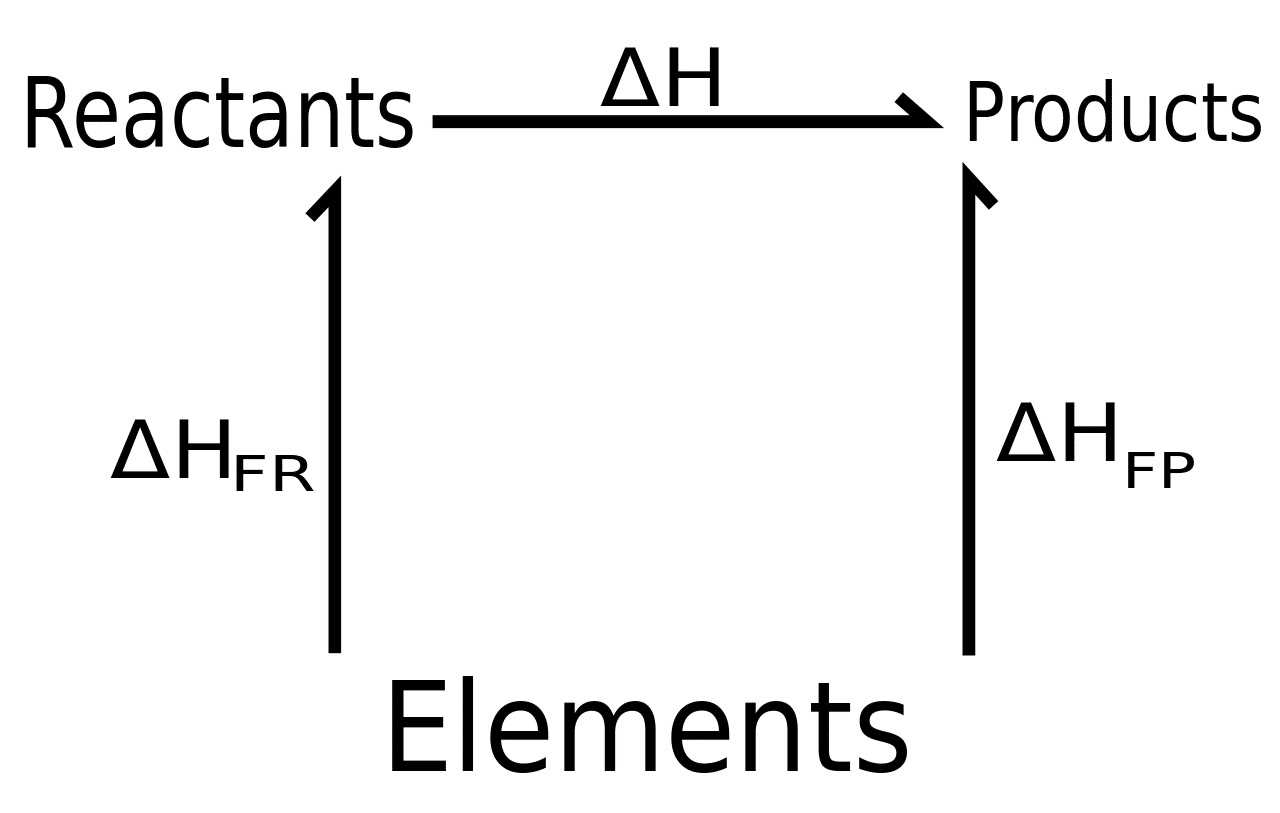
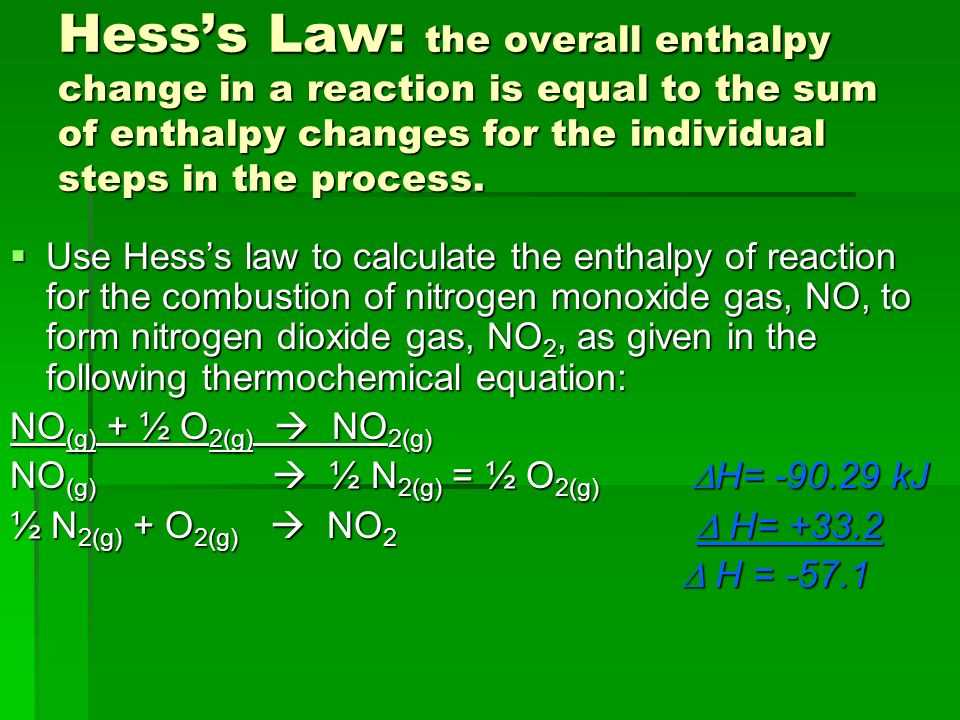
Hess’s Law Explained Simply
At the heart of many chemical processes is the principle that energy changes are independent of the pathway taken, as long as the initial and final conditions remain the same. This idea simplifies the way we understand how energy is transferred during transformations. Instead of measuring every step in a complex process, we can focus on the overall change in energy, making the analysis more manageable and efficient.
This concept can be broken down into a few key points:
- Energy Change is Pathway Independent: The total energy change of a system only depends on the starting and final states, not on the path the process takes.
- Multiple Steps Can Be Combined: If a transformation occurs in several stages, the total energy change is the sum of the changes in each step, allowing for easier calculations.
- Helps in Complex Calculations: By applying this idea, complex processes that would be difficult to measure directly can be understood through simpler, indirect measurements.
This principle is incredibly useful for simplifying calculations and predicting energy changes in processes that are too complicated to observe directly. It serves as a fundamental tool in both research and practical applications, especially in situations where energy shifts need to be precisely measured but the process itself cannot be easily analyzed in real-time.
How Enthalpy Relates to Hess’s Law

The connection between energy changes during a transformation and how they can be measured through different stages is crucial for understanding many processes. By observing how energy is absorbed or released, we can predict the outcome of a complex event based on simpler, known steps. This approach not only simplifies analysis but also provides a deeper insight into the fundamental principles that govern chemical changes.
Energy Change Through Multiple Stages
One way to understand this relationship is by considering how a series of transformations can be combined to determine the overall energy change. Instead of directly measuring each step, it is possible to add up the individual changes to calculate the total shift. This makes it easier to analyze multi-step processes and calculate energy fluctuations without the need for extensive experiments.
- Stage-wise Energy Calculation: Energy shifts in each phase are added together to find the total change in a process, regardless of the path taken.
- Predicting Overall Energy: If individual changes are known, the final energy change can be calculated, even if the complete process is not directly observable.
- Importance of Consistency: The energy change remains the same, whether measured in one step or several, as long as the initial and final conditions are consistent.
Practical Applications in Chemical Processes
This relationship is incredibly valuable when dealing with chemical processes that are too complex to measure directly. By breaking down these processes into smaller, more manageable parts, researchers can easily determine the total energy change, saving time and resources. This is particularly useful in both experimental settings and industrial applications where precise energy measurements are needed to control or optimize reactions.
Calculating Heat Changes in Reactions
Understanding how heat is exchanged during chemical transformations is essential for predicting the behavior of substances under various conditions. By calculating the energy absorbed or released, scientists can gain valuable insights into the nature of the process. This ability to quantify heat shifts allows for better control and optimization of processes in both experimental and industrial settings.
Methods for Calculating Heat Changes
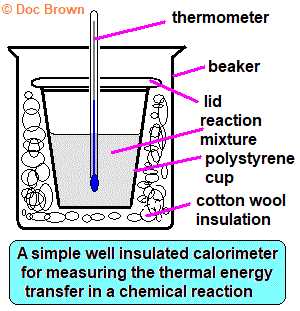
There are several methods used to calculate the energy shifts that occur during transformations. These methods typically involve measuring temperature changes and applying specific formulas to determine the amount of heat transferred. One of the most common approaches is the use of calorimetry, where a substance is placed in a calorimeter, and the temperature change is measured to calculate the heat involved.
- Calorimetry: This technique involves measuring the heat change by observing the temperature shift in a known substance or system, often using a calorimeter.
- Specific Heat Capacity: By knowing the specific heat of a substance and its mass, the heat change can be calculated using the formula Q = mcΔT, where Q is the heat, m is the mass, c is the specific heat, and ΔT is the temperature change.
- Bond Energy Calculations: In some cases, the heat change can be determined by considering the energy required to break or form chemical bonds in the reactants and products.
Interpreting Heat Changes
Once the heat change is calculated, it is important to interpret what this means for the process at hand. A positive heat change typically indicates that energy was absorbed, suggesting that the process is endothermic. Conversely, a negative heat change implies that energy was released, signifying an exothermic process. Understanding these shifts can help predict how a system will behave under different conditions and guide experimental design to achieve desired results.
Application of Hess’s Law in Reactions
The ability to determine energy changes in complex processes by breaking them down into simpler steps is invaluable in both research and industry. By applying a specific principle, scientists can calculate the overall energy shift in a multi-step process, even when direct measurement is not feasible. This approach simplifies the study of energy dynamics in various transformations and provides a reliable method for predicting the outcomes of chemical processes.
One of the most significant applications of this principle is in the synthesis of compounds or the study of reactions that involve intermediate steps. By combining known energy changes for individual stages, researchers can determine the total energy change without needing to observe each step directly. This is particularly useful in cases where the transformation involves reactions that are difficult or impractical to study in real-time.
Additionally, this method plays a crucial role in the design of industrial processes, where controlling energy efficiency is key. By understanding how energy is transferred during each step, engineers can optimize conditions to minimize energy loss, improve reaction rates, and reduce the overall cost of production. This knowledge also allows for the development of new materials and products by predicting how different substances will interact under specific conditions.
Step-by-Step Approach to Pre-Lab Preparation
Effective preparation is key to ensuring a smooth and successful experiment. The process involves understanding the objectives, reviewing the necessary procedures, and organizing the required materials. By carefully planning ahead, you can minimize errors, save time, and gain a deeper understanding of the concepts behind the experiment.
Follow these essential steps for optimal preparation:
- Review the Objective: Start by reading the purpose of the experiment thoroughly. Ensure you understand the overall goals and what you are trying to measure or observe during the process.
- Understand the Procedure: Familiarize yourself with the experimental steps. Pay attention to key instructions and any important safety precautions. If there are multiple stages, make sure you understand the sequence and how they relate to each other.
- Gather Materials: Organize all the tools, chemicals, and equipment you will need. Check that everything is in proper working condition and that you have the correct quantities.
- Calculate Necessary Values: If the experiment involves specific measurements, perform any calculations in advance. This could include determining quantities of reagents, expected temperatures, or other relevant data.
- Review Related Concepts: Brush up on the theoretical background related to the experiment. Understanding the scientific principles will help you interpret results and understand why certain steps are performed.
Taking the time to thoroughly prepare will ensure that you are confident and well-equipped for the experiment, helping you focus on the process itself rather than troubleshooting or catching up with missing steps.
Common Mistakes in Enthalpy Calculations

Accurate energy calculations are crucial for understanding the behavior of substances during transformations. However, even small errors can lead to significant discrepancies in the results. Common mistakes often arise from misinterpreting formulas, overlooking key factors, or improper data handling. Recognizing these pitfalls can help avoid inaccuracies and ensure more reliable outcomes in your experiments.
Here are some of the most frequent mistakes to watch out for when performing energy-related calculations:
| Error | Cause | How to Avoid |
|---|---|---|
| Incorrect Units | Mixing up units like joules with kilojoules or grams with kilograms. | Always check units before performing calculations and ensure consistency throughout. |
| Wrong Formula Application | Using the incorrect formula for heat calculation or confusing specific heat with other variables. | Understand the formula’s context and confirm that all variables are correctly applied to the equation. |
| Overlooking Heat Losses | Not accounting for heat losses to the surroundings during the process. | Ensure all heat exchanges, including those lost to the environment, are considered in the calculation. |
| Misreading Temperature Changes | Incorrectly recording the temperature change or confusing the initial and final temperatures. | Use accurate thermometers and carefully track the temperature changes to avoid errors. |
| Failure to Use Correct Data | Using outdated or incorrect values for constants like specific heat or molar mass. | Always use the most current and accurate data, checking values from reliable sources before using them. |
By being mindful of these common errors, you can improve the accuracy of your calculations and achieve more reliable results in your experiments.
Experimental Techniques for Measuring Enthalpy
Measuring energy changes during a physical or chemical process requires precise techniques to ensure accurate results. Various methods exist, each with specific applications depending on the experiment’s nature and the materials involved. These techniques rely on monitoring temperature changes, heat flow, or pressure variations, and choosing the right approach is key to obtaining reliable data.
Below are some commonly used methods to measure energy shifts in experiments:
- Calorimetry: This technique measures heat changes by monitoring the temperature variation of a sample in response to energy transfer. A calorimeter is used to isolate the system and accurately measure temperature changes, allowing for energy calculations.
- Bomb Calorimetry: Often used for combustion reactions, this method involves igniting a substance in a sealed chamber and measuring the temperature rise in a known mass of water. This provides precise data for calculating energy changes in reactions involving fuels.
- Differential Scanning Calorimetry (DSC): This technique monitors the heat flow into or out of a sample as it is heated or cooled. The difference in heat flow between the sample and a reference material is recorded to determine energy absorption or release during phase changes.
- Constant Pressure Method: In this approach, the process occurs at constant pressure, and the heat change is measured by observing the temperature change in the surrounding environment or a specific medium like water.
- Thermogravimetric Analysis (TGA): This method is used to study the mass changes of a substance as it is heated, which can correlate to energy changes. This is particularly useful for reactions involving material decomposition or evaporation.
Each of these methods has its advantages and is suited for different types of experiments. Choosing the appropriate technique ensures more accurate measurements, which are essential for understanding the energy dynamics of the system under study.
Importance of Accuracy in Thermodynamics Labs
In scientific experiments, particularly those dealing with energy transformations, precision is crucial. Small errors in measurements can lead to significant discrepancies in results, potentially affecting the overall understanding of the system being studied. Ensuring high accuracy allows researchers to draw valid conclusions and make reliable predictions based on experimental data.
When conducting experiments that involve heat flow or energy exchange, even minor deviations can have a noticeable impact on the calculations. Accurate temperature readings, precise calibration of instruments, and careful handling of materials all play a role in achieving trustworthy results. Without attention to detail, it becomes difficult to interpret outcomes or validate theoretical models.
The following points illustrate why precision is critical:
- Consistency: Reproducible results are only possible when measurements are taken with accuracy. This ensures that experiments can be repeated with similar outcomes, reinforcing the validity of the results.
- Reliability of Data: Accurate data is the foundation of scientific analysis. When calculations are based on reliable figures, predictions made from the data are more likely to be correct and useful for future research.
- Reducing Error Margins: Small errors in temperature or pressure measurements can lead to large discrepancies when calculating energy changes. Minimizing these errors increases the precision of calculated values, making conclusions more dependable.
- Validation of Models: Theoretical models and calculations are often based on experimental data. If the experimental results are inaccurate, the models may no longer accurately represent real-world systems, leading to flawed predictions or misinterpretations.
- Optimizing Efficiency: In many cases, understanding energy changes helps optimize processes, whether for industrial applications or academic research. Accurate measurements allow for better efficiency, reducing waste and improving the overall design of experiments or systems.
In conclusion, ensuring precision in energy measurements is essential for producing valid results, testing hypotheses, and advancing scientific knowledge. Accuracy is not just a technical requirement; it is a fundamental aspect of the scientific method that shapes how we understand and interact with the physical world.
Relation Between Enthalpy and Temperature
The connection between heat content and temperature is a fundamental concept in studying energy changes within systems. As temperature fluctuates, the internal energy of a system is affected, influencing the overall energy state. This relationship is central to understanding how energy transfers and transforms in various physical processes, whether during heating, cooling, or phase changes.
When the temperature of a system increases, the average kinetic energy of the particles within it also rises. This causes more vigorous molecular motion, often leading to an increase in the system’s heat content. Conversely, when the temperature drops, molecular motion slows, and the overall heat within the system decreases. The way temperature influences energy content is crucial for understanding how heat flows in or out of a system during different processes.
The following factors are important in understanding how temperature relates to the energy content of a system:
- Heat Transfer: When energy is added to or removed from a system, the temperature changes. This change affects the energy balance and can indicate whether a process is endothermic or exothermic.
- Specific Heat Capacity: Different substances require varying amounts of energy to change their temperature. The specific heat capacity determines how much energy is needed to raise or lower the temperature of a material by a given amount.
- Phase Changes: During phase transitions, such as from solid to liquid or liquid to gas, temperature changes do not always correlate directly with changes in energy content. Energy is used to overcome intermolecular forces rather than changing temperature during these processes.
- Thermodynamic Processes: In processes like expansion or compression, temperature plays a key role in the system’s behavior. For example, in an isothermal process, temperature remains constant, but energy is still transferred to or from the system.
Understanding the link between temperature and energy changes is essential for predicting how systems behave under different conditions. Whether in chemical engineering, physics, or environmental science, this relationship is a core principle for designing systems and analyzing the flow of energy.
Practical Examples of Hess’s Law
Understanding the relationship between energy changes in a system can be greatly enhanced by applying principles of heat transfer in practical scenarios. One of the most important concepts is that energy changes in a system can be determined through indirect pathways, provided certain conditions are met. By combining multiple energy processes, the total energy change can be computed accurately, even if direct measurement is not possible. Below are a few real-world applications that demonstrate how this concept works in various fields.
Example 1: Combustion of Carbon
One of the most common examples is the combustion of carbon. To determine the total heat released during the combustion of carbon in oxygen, one can apply indirect methods. The process involves burning carbon in oxygen to form carbon dioxide, which can be broken down into simpler steps. These individual steps, when combined, help in calculating the total energy change, even if the reaction itself cannot be measured directly in a single experiment.
| Step | Process | Energy Change (kJ) |
|---|---|---|
| 1 | Combustion of Carbon to form Carbon Monoxide | -110.5 |
| 2 | Combustion of Carbon Monoxide to form Carbon Dioxide | -283.0 |
| Total | Combustion of Carbon to form Carbon Dioxide | -393.5 |
Example 2: Formation of Water from Hydrogen and Oxygen
Another classic example is the formation of water from hydrogen and oxygen gases. This process is highly exothermic, and by applying indirect methods, the heat released can be determined by combining the formation of hydrogen from its elements and the formation of water from hydrogen and oxygen. The overall energy change is calculated by summing the energy changes of each individual step.
| Step | Process | Energy Change (kJ) |
|---|---|---|
| 1 | Formation of Hydrogen from Elements | 0 |
| 2 | Formation of Water from Hydrogen and Oxygen | -241.8 |
| Total | Formation of Water from Hydrogen and Oxygen | -241.8 |
These examples highlight how energy changes can be understood and calculated using indirect methods. This technique is especially useful in experiments where direct measurement is challenging, or when complex processes are involved. Through these practical applications, it becomes clear that understanding energy transfer is crucial in various scientific and industrial contexts.
Challenges in Enthalpy and Reaction Studies
Understanding the energy changes in chemical processes is a complex task that presents several challenges. While the principles behind energy transfer are well-established, measuring these changes accurately in real-world conditions can be difficult. Several factors influence the precision and reliability of data, making it essential for researchers to carefully design their experiments and account for variables that could distort their findings. Below are some of the key challenges faced during studies focused on energy shifts in processes.
1. Heat Loss to the Environment
One of the most significant difficulties in these types of studies is minimizing heat exchange with the surroundings. In an ideal scenario, all the heat produced or absorbed would be contained within the system. However, in practice, some of the energy will inevitably escape to the environment, leading to inaccurate measurements. This is especially true for experiments conducted in open containers or under non-ideal conditions, where the heat dissipation cannot always be controlled.
2. Incomplete or Side Processes
Another challenge is ensuring that only the intended processes are occurring during an experiment. Chemical systems are often subject to side reactions or incomplete conversions, which can contribute additional energy changes that are not part of the main reaction being studied. These unintended processes can introduce errors, making it difficult to attribute the observed energy changes to the reaction of interest.
Furthermore, certain reactions may take place in stages, with energy being absorbed or released at different points. If the experiment does not capture every step in the process or fails to account for all intermediate states, the energy calculations may not be accurate. Understanding the reaction mechanisms thoroughly is critical to designing experiments that isolate the desired processes.
3. Instrumentation Limitations

Accurate measurement of energy changes often relies on sophisticated equipment, such as calorimeters, which can be sensitive to minor fluctuations. These instruments must be calibrated properly to avoid systematic errors. Even small calibration errors can lead to substantial inaccuracies in data, especially when the energy changes being measured are relatively small. In addition, some reactions release or absorb energy too rapidly for certain instruments to measure accurately, posing another obstacle for researchers.
Despite these challenges, researchers continue to refine their techniques and instrumentation to improve the reliability of energy measurements. By recognizing and addressing these potential issues, they can gain more accurate insights into the energy dynamics of chemical processes, which is crucial for advancing scientific understanding and technological applications.
Impact of Catalyst on Enthalpy Changes
Catalysts play a critical role in chemical processes by influencing the speed at which they occur, but they do not directly alter the energy changes associated with these processes. While a catalyst can lower the activation energy required for a process to proceed, it does not change the overall energy difference between reactants and products. This section explores the effect of catalysts on energy shifts in systems and their implications for both the rate and magnitude of these changes.
1. Catalyst’s Role in Energy Activation
The primary function of a catalyst is to provide an alternative pathway for a process with a lower activation energy. By facilitating this easier path, a catalyst helps molecules overcome the energy barrier more efficiently. However, it is essential to note that while this acceleration is significant, it does not change the total energy involved in the system. The energy released or absorbed as a result of the process remains the same as it would without the catalyst, as the catalyst does not consume energy itself in the process.
2. No Impact on Overall Energy Change
Although a catalyst speeds up a process, it does not affect the total energy difference between the starting materials and the end products. Whether a catalyst is used or not, the amount of energy released or absorbed by the system is determined by the nature of the substances involved, not by the presence of the catalyst. The key takeaway is that a catalyst alters the rate of energy flow but does not change the extent of energy transformation during the process.
Thus, while catalysts are invaluable tools in enhancing the efficiency of chemical processes, they should not be expected to influence the overall energy balance of a system. Their primary contribution is in reducing the time needed for the process to reach completion, which is critical in industrial applications and experimental setups.
Understanding Standard Enthalpy of Formation
The concept of the standard energy change during the creation of compounds from their basic elements is essential for understanding the dynamics of chemical processes. This value is a fundamental component in calculating energy exchanges in various systems. By evaluating the energy required to form a substance from its constituent elements under standard conditions, it becomes possible to predict and explain the energy profiles of more complex processes.
1. Definition and Importance
The standard energy change for forming a compound is typically measured under standard conditions (298 K, 1 atm pressure). This value is important because it serves as a benchmark for evaluating other processes. By knowing the energy associated with the formation of a compound, one can estimate the heat exchanges that would occur in real-life reactions where these compounds are involved. The process allows for a deeper understanding of both energy absorption and release during compound formation.
2. Calculating Energy Changes from Formation Values
To calculate the total energy change of a process, one can sum the standard energy changes of formation for each component in the system. This can be done using a combination of the following methods:
- Direct measurement of formation energy under controlled conditions
- Using tabulated values of standard energy changes for common compounds
- Employing stoichiometric methods to determine the overall change based on reactants and products
These calculations are useful in various fields, from industrial chemistry to environmental studies, where understanding energy changes is crucial for optimizing efficiency and sustainability.
3. Example Table: Standard Formation Energy of Common Compounds
| Compound | Standard Energy of Formation (kJ/mol) |
|---|---|
| Water (H2O) | -241.8 |
| Carbon Dioxide (CO2) | -393.5 |
| Oxygen (O2) | 0 |
| Nitrogen (N2) | 0 |
The values in this table highlight the energy required or released when these compounds are formed from their elemental components in their most stable states. For example, the formation of water from hydrogen and oxygen releases a significant amount of energy, indicating its stability as a compound.
Real-World Applications of Hess’s Law
The principles that govern energy changes during processes are not limited to theoretical studies; they play a crucial role in numerous practical applications across industries. Understanding how energy behaves during transformations can help scientists, engineers, and chemists optimize processes, improve efficiency, and predict outcomes. Below are several areas where these principles are applied to solve real-world problems.
1. Chemical Manufacturing

In the production of chemicals, understanding energy transformations is vital for designing processes that minimize energy consumption. By calculating the energy needed for various stages of production, manufacturers can:
- Optimize heat exchange systems
- Reduce waste by controlling energy inputs and outputs
- Lower costs by designing more efficient chemical synthesis pathways
For example, the synthesis of ammonia from nitrogen and hydrogen, a key step in fertilizer production, requires precise energy calculations to ensure that the process is both economically and environmentally efficient.
2. Environmental Impact Assessments
In environmental science, understanding how energy is absorbed or released during natural or artificial processes helps predict the long-term effects of various activities, such as the burning of fossil fuels or the degradation of materials. By knowing the heat changes involved in these processes, researchers can:
- Assess the carbon footprint of different industrial operations
- Develop strategies to reduce the environmental impact of manufacturing and transportation
- Analyze the long-term sustainability of alternative energy sources
This knowledge is also applied in the design of green technologies, such as energy-efficient buildings or renewable energy systems, where minimizing energy waste is critical.
3. Pharmaceuticals and Drug Development
In drug design, understanding energy changes is essential for creating stable compounds. Scientists use energy calculations to:
- Predict the stability of chemical compounds
- Identify the most efficient synthesis routes for new drugs
- Ensure that drugs maintain their potency over time
By applying these calculations, pharmaceutical companies can reduce costs and improve the quality of their products while meeting regulatory standards for safety and efficacy.
4. Energy Efficiency in Engineering
In the field of engineering, especially in the design of engines, turbines, and heat exchangers, energy transformation calculations are crucial. Engineers use these principles to:
- Maximize the efficiency of thermal engines, such as those in power plants or vehicles
- Improve the design of heat management systems to minimize energy loss
- Develop new technologies that use alternative energy sources more efficiently
By understanding how energy is transferred during mechanical processes, engineers can design systems that conserve energy, improve performance, and reduce environmental impact.
5. Food Industry
The food industry also benefits from these principles. Energy calculations are used in the design of cooking processes, preservation methods, and packaging technologies. For example:
- Energy inputs are carefully calculated to determine the most efficient ways to preserve food without compromising taste or nutritional value.
- Energy is also considered in processes like pasteurization, where controlling temperature is critical to maintaining food safety.
Understanding how energy is absorbed and released during these processes helps optimize the production, storage, and transportation of food products.