
In chemistry, observing the color changes produced by various substances when heated is an essential method for identifying different elements. These reactions not only demonstrate the unique properties of elements but also provide valuable insights into their atomic structure and behavior. By carefully analyzing these reactions, students and researchers can accurately determine the presence of specific metals in a sample.
This experiment involves heating compounds and observing the colors emitted, which are characteristic of the elements involved. Each element produces a distinct hue when exposed to high temperatures, allowing for quick identification. Understanding these reactions is fundamental for anyone studying inorganic chemistry, as it provides a practical and visual approach to recognizing key elements.
Through this section, you will explore various methods of interpreting the results, along with a detailed breakdown of the color reactions for common metals. The information provided here serves as a guide to understanding these processes and helps ensure accurate identification when performing this hands-on exercise in a controlled setting.
Flame Test Lab Answer Key Overview
In this section, we will explore the key outcomes and observations from the experiment, which involves heating various compounds to observe the characteristic colors produced by different elements. This process is essential for understanding how metals behave when subjected to heat and how their unique signatures can be used for identification.
Purpose of the Experiment

The primary goal is to recognize the relationship between an element’s atomic structure and the color it emits when exposed to high temperatures. By studying this phenomenon, one can accurately identify metals and their compounds in an unknown sample. This visual technique is a valuable tool for chemists and researchers working with metal analysis.
Key Observations and Reactions
Here are some typical reactions and the colors associated with each metal compound:
- Sodium: Bright yellow
- Potassium: Lilac or light purple
- Calcium: Orange-red
- Copper: Green or blue
- Barium: Green
By understanding these color patterns, students can correctly match a specific element with its corresponding reaction. The process is crucial for accurately determining the composition of an unknown substance based on its visible response to heat.
Purpose of the Flame Test Experiment
The main objective of this procedure is to observe the characteristic color changes produced when different metals are heated. These color changes occur due to the excitation of electrons within the atoms of the elements, and they serve as a key indicator for identifying substances based on their unique emission spectra. This technique is widely used in chemistry to study the properties of elements and their compounds.
By examining the light emitted during the heating process, students and researchers can learn to identify unknown metals or chemical compounds, aiding in chemical analysis and laboratory identification. The colors produced are distinctive for each element and can be used as a diagnostic tool to confirm or differentiate metals present in a sample.
Key Goals of the Experiment
- Identification of elements: Recognize specific metals based on their characteristic colors when exposed to heat.
- Understanding atomic structure: Observe how electrons within atoms interact with energy and produce visible light.
- Application in chemical analysis: Use color emissions as a simple and effective method for substance identification.
Overall, this experiment provides a practical approach to understanding both the theoretical and practical aspects of elemental behavior when subjected to high temperatures, making it a valuable educational tool in chemistry.
Understanding Flame Colors and Their Significance
When substances are exposed to heat, they often emit a characteristic light, with each element producing a unique color. This occurrence happens because the heat excites the atoms within the material, causing their electrons to jump to higher energy levels. When these electrons return to their original state, they release energy in the form of light. The color of this light is directly related to the energy transitions of the atoms involved. The ability to observe and identify these colors provides valuable information about the elements present in the material.
Each element has a specific emission spectrum, which is a set of distinct colors it produces under certain conditions. These colors are used by scientists to identify substances and understand their chemical composition. By carefully analyzing the light emitted, researchers can determine which elements are present in the sample, making this technique an essential tool in both academic and practical scientific applications.
Common Elements and Their Emission Colors
| Element | Color Emitted |
|---|---|
| Sodium | Bright yellow |
| Potassium | Light purple |
| Calcium | Orange-red |
| Copper | Green or blue |
| Barium | Green |
The specific colors produced by different elements when heated are essential for identifying them in various scientific experiments. By observing the color of the light emitted, researchers can rapidly identify the chemical composition of unknown samples. This technique is widely used in fields like chemistry, environmental science, and materials analysis, where quick identification of elements is crucial for accurate research and analysis.
Common Elements Tested in Flame Test

Several substances are frequently analyzed using heat-based methods to identify the elements they contain. These materials are chosen because they exhibit distinct colors when heated, which allows for quick and easy identification. By observing the emitted light, scientists can determine the presence of specific elements in a sample. The following elements are commonly studied for their unique emission colors, making them useful in a variety of scientific applications.
Sodium and its Bright Yellow Hue
Sodium is one of the most recognizable elements when subjected to heating. It emits a vivid yellow color, which is easily detected. This strong and clear signal makes sodium a common target for this type of analysis. It is often used in experiments where the goal is to observe the behavior of common metals under heat.
Potassium and its Soft Violet Glow

Potassium is another element that provides a distinctive color when heated. Its emission is a soft violet or lavender shade, which is useful for distinguishing it from other elements. The subtlety of its color makes it an ideal candidate for identifying potassium in complex mixtures.
Other Notable Elements
- Calcium: Emits an orange-red light, which is easily distinguishable from other metal emissions.
- Copper: Known for its green to blue emission, copper is often analyzed for its vibrant colors.
- Barium: Produces a green color when heated, which is also used for identification in various scientific fields.
These elements are commonly examined using heat-based analysis techniques due to their ability to produce distinct colors. This simple method provides valuable insights into the composition of materials, helping researchers identify substances quickly and efficiently. The ability to recognize these colors is critical for applications ranging from chemistry to environmental monitoring, where precise identification of materials is essential.
How to Conduct the Flame Test Lab
Performing this procedure involves heating various materials to observe their characteristic light emissions. By introducing specific substances into a hot environment, you can witness unique colors emitted by each element, allowing for identification. The process is relatively straightforward but requires careful attention to safety and technique to ensure accurate results and avoid contamination of the samples.
Required Materials and Setup

To perform this experiment, you’ll need the following materials:
- A Bunsen burner or heat source
- Clean metal loops or wire to hold samples
- Several test samples containing different metal salts or compounds
- A heat-resistant surface or stand
- Safety equipment such as goggles and gloves
Before starting the experiment, ensure that the workspace is clean and free of any flammable materials other than those intended for testing. The metal loops should be thoroughly cleaned between each sample to avoid cross-contamination and inaccurate results.
Procedure for Conducting the Experiment
Follow these steps carefully to observe the emission colors:
- Prepare the metal loop by cleaning it with distilled water and a flame to ensure no previous residue remains.
- Dip the loop into the sample solution you wish to analyze.
- Hold the loop in the flame, ensuring that the material is heated thoroughly, and observe the color that is emitted.
- Record the color emitted by the substance. Each metal will produce a distinct color when heated, which can be compared to a reference chart.
- Repeat the process for different samples, cleaning the loop between each to avoid cross-contamination.
By following these steps, you can successfully identify various elements based on the colors they emit when subjected to high heat. This method provides an easy and effective way to understand the relationship between the physical properties of materials and their chemical composition.
Key Chemical Reactions in Flame Tests
The process of observing the colors produced when substances are exposed to intense heat is rooted in fundamental chemical reactions. These reactions occur as metal ions are excited by the energy from the heat, causing them to emit light at specific wavelengths. The colors emitted during these reactions are characteristic of the element or compound being tested, providing insight into the chemical composition of the substance. Understanding these reactions is crucial for interpreting the results accurately and can be applied to identify unknown elements in various compounds.
Excitation and Relaxation of Electrons
The core reaction that occurs in these procedures involves the excitation of electrons within metal atoms. When a metal salt or compound is heated, the energy from the heat excites the electrons, pushing them to higher energy levels. As the excited electrons return to their original or lower energy levels, they release the excess energy in the form of light. The specific wavelength (or color) of this light is determined by the difference in energy between the excited and relaxed states of the electrons. This is why each metal produces a unique color when subjected to high heat.
Common Element Reactions
Different metals and metal compounds produce distinctive colors due to the specific wavelengths of light they emit during electron transitions. Some common reactions include:
- Sodium: Produces a bright yellow color due to the emission of light in the wavelength range of approximately 589 nm.
- Potassium: Produces a pale violet or lilac color, which is a result of the emission at around 404 nm.
- Copper: Produces a green or blue-green color due to transitions in its electron shell.
- Calcium: Emits an orange-red color, commonly observed in calcium salts.
- Barium: Produces a green color when heated, often used for identifying barium compounds.
Each of these reactions provides a clear visual indicator of the presence of specific elements. By carefully observing the emitted colors, it is possible to identify the components of a sample with high precision, making this technique an invaluable tool in both educational and professional chemistry applications.
Interpreting Flame Colors for Identifying Metals
The observation of specific colors emitted when metals are heated plays a crucial role in identifying different elements. The heat excites electrons within metal atoms, causing them to release light at specific wavelengths, which manifest as distinct colors. These colors can be used as a reliable method for determining the presence of particular metals in a substance. Each metal emits a unique color pattern when exposed to high temperatures, allowing chemists to identify them based on these characteristics.
By analyzing the emitted color, one can deduce the metal present in a sample. This method is widely used in various scientific fields, including chemistry and forensic science, as it provides a simple and effective way to detect the composition of unknown materials. Understanding the relationship between metal types and their corresponding flame colors is essential for accurate identification.
Common Metals and Their Corresponding Flame Colors
Here is a list of common metals and the characteristic colors they produce when heated:
- Sodium: Emits a bright yellow color, one of the most easily recognizable hues produced in heating reactions.
- Potassium: Produces a pale violet or lavender color, which can sometimes be difficult to distinguish from other colors but is still distinctive.
- Copper: Produces a green or blue-green color, which is unique and helpful in identifying copper compounds.
- Calcium: Emits a strong orange-red color, often seen in various calcium salts.
- Barium: Emits a vibrant green color, typically associated with barium-containing compounds.
- Strontium: Produces a bright red color, often used in fireworks and other pyrotechnic applications.
- Litium: Gives a crimson or deep red hue when exposed to high temperatures.
By comparing the color emitted by a heated substance with known standards, one can confidently identify the metal present in a sample. This technique is not only useful in the laboratory but also in real-world applications such as metallurgy, chemical analysis, and even in the study of materials in forensic investigations.
Flame Test Answer Key: Key Observations
When conducting experiments involving the heating of metal salts, several important observations can provide valuable insights into the composition of the substances. These observations typically focus on the color emitted during the process, as different elements release distinct colors when heated. Understanding these color patterns is essential for accurately identifying the metals present in a sample.
For example, the intense yellow glow seen during the heating of sodium compounds is a clear indicator of the presence of sodium. Similarly, lithium salts emit a red color, which is often used to distinguish lithium from other metals. The unique hues produced by various metals can be a helpful diagnostic tool, and careful note-taking of these observations is key to drawing accurate conclusions about the identity of the substances involved.
In addition to color, the intensity of the emitted light can also provide useful information. Some metals may produce a very bright or vivid color, while others may emit a more subtle glow. These variations in intensity can further refine the identification process, allowing for a more precise understanding of the material being tested.
Overall, the ability to observe and interpret these color changes is crucial for successful identification and analysis in various scientific fields, including chemistry and materials science. By documenting the colors and their corresponding metals, researchers can build a comprehensive understanding of the substances they are working with, leading to more accurate and reliable results in their experiments.
Safety Precautions for Flame Test Labs
When conducting experiments involving high temperatures and chemical reactions, it is essential to prioritize safety to prevent accidents and injuries. Handling heating devices and chemicals requires careful attention to detail and adherence to proper protocols to ensure that risks are minimized. Following safety guidelines is not only important for the well-being of the individuals performing the experiments, but also for the integrity of the results obtained.
First and foremost, all participants should wear appropriate personal protective equipment (PPE), including safety goggles, lab coats, and heat-resistant gloves. These items help protect against potential exposure to harmful substances and high temperatures. In addition, it is crucial to ensure that the working environment is well-ventilated to avoid inhaling any fumes that may be released during the heating process.
Furthermore, all chemicals should be handled with caution. It is important to use proper containers for storing and mixing chemicals, and care should be taken to avoid spills and splashes. Always check the material safety data sheets (MSDS) for each substance used to understand the specific hazards involved. In the case of any spills, immediately follow the appropriate cleanup procedures outlined in the safety protocols.
It is also recommended to have a fire extinguisher, eyewash station, and first aid kit readily available in case of emergencies. Before starting any experiment, review all safety instructions and ensure that all equipment is in good working order. Avoid distractions and never leave heating equipment unattended. These simple precautions can go a long way in preventing accidents and ensuring a safe experimental environment.
Factors Affecting Flame Test Results
The outcomes of experiments involving the observation of color emissions can be influenced by several variables. Understanding these factors is essential for accurate analysis and interpretation. Different elements and compounds react in various ways when subjected to heat, leading to distinct patterns of light emissions. However, external conditions and the method of conducting the experiment can also play a significant role in the observed results.
1. Temperature Control

One of the most important factors affecting the results is the precise control of temperature during the heating process. Fluctuations in temperature can lead to inconsistencies in the emission of light, altering the color produced. The intensity of the heat can also influence the sharpness and clarity of the observed colors. Therefore, maintaining a stable temperature is crucial for obtaining reliable results.
2. Purity of the Sample
The composition of the sample being heated is another key element. Contaminants or impurities present in the sample can affect the quality and accuracy of the color produced. Even trace amounts of other substances can interfere with the desired outcome, as they may introduce additional emissions or alter the overall reaction. To ensure accuracy, it is important to use as pure a sample as possible.
Other factors such as the type of heating apparatus, the surrounding environment, and even the presence of other gases or materials in the air can influence the final results. Recognizing these factors and taking the necessary precautions can greatly improve the reliability of the experiment and the clarity of the results.
Color Observations for Sodium
Sodium is one of the most commonly examined elements when it comes to identifying compounds based on their emission characteristics under heat. The unique color it emits when heated can be easily recognized and is used for confirming the presence of sodium in a sample. The distinct yellow hue it produces makes sodium a key element in many chemical demonstrations. Understanding the expected color and its significance allows for accurate identification of sodium-containing substances in various settings.
Color Emission
When sodium salts are exposed to intense heat, they typically produce a vibrant yellow color. This bright yellow emission is one of the most distinctive features used to identify sodium during experiments. The intensity and clarity of the yellow light can vary based on factors such as the concentration of sodium in the sample and the temperature of the heating process.
Common Sodium Compounds

Sodium chloride (NaCl) and sodium carbonate (Na2CO3) are among the most frequently tested sodium compounds. Both of these substances will exhibit the characteristic yellow emission when heated. While other elements might also produce colorful emissions, the yellow glow from sodium is unique and easily distinguishable, making it a reliable indicator of sodium’s presence in a sample.
Color Observations for Potassium
When heated, potassium compounds emit a distinctive color that is commonly used to identify the presence of potassium in various substances. The color emitted by potassium is unique and can be easily distinguished from other elements during a heating process. Understanding this characteristic emission is essential for confirming the presence of potassium in chemical samples. The purple or lilac color observed under heat is a key indicator of potassium salts and is widely utilized in both educational and industrial applications.
Color Emission
Potassium compounds typically produce a pale lilac or light purple color when exposed to intense heat. This specific hue is one of the defining features used to identify potassium in a mixture. The purple color is often soft and can sometimes appear faint, depending on the concentration of potassium in the sample and the intensity of the heating process. However, the distinct shade is still noticeable and can be reliably used to identify potassium in various mixtures.
Common Potassium Compounds
Potassium chloride (KCl) and potassium nitrate (KNO3) are common compounds that exhibit the characteristic purple color when heated. These potassium salts are frequently tested in experiments to observe their unique color emission. The lilac color produced by these compounds is a reliable indicator of the presence of potassium, making it easy to distinguish from other elements that emit different colors under similar conditions.
Color Observations for Calcium
When calcium compounds are exposed to high temperatures, they emit a specific color that is useful for identifying the presence of calcium in a substance. This particular color is a defining characteristic that allows chemists and researchers to distinguish calcium from other elements. The emitted color is bright and easily identifiable, making it an essential tool for chemical analysis and identification in various applications.
Color Emission
Calcium compounds typically produce a brick-red or orange-red color when heated. This warm, distinct hue is easily observable and serves as a reliable indicator of calcium’s presence. The intensity of the color may vary depending on the concentration of calcium in the sample and the temperature used, but the overall brick-red tone remains the key identifying feature of calcium salts under heat.
Common Calcium Compounds
Calcium chloride (CaCl₂) and calcium nitrate (Ca(NO₃)₂) are two common compounds that exhibit the characteristic red-orange color when heated. These calcium salts are frequently used in demonstrations and experiments, where their color emission can be clearly observed. The distinct brick-red color produced by these compounds provides a straightforward method for identifying calcium in a mixture or sample.
Color Observations for Copper
Copper salts are known to produce distinctive visual effects when subjected to high heat, which can be utilized for their identification. The color emitted by copper compounds helps in distinguishing them from other elements and offers a simple yet effective method for analysis. This characteristic color is often used in both educational demonstrations and scientific experiments to reveal the presence of copper in a substance.
Color Emission
When copper compounds are heated, they typically emit a green or blue-green color. This hue can range from a light turquoise to a deeper emerald shade, depending on the specific copper compound being used. The greenish color is the key visual indicator for copper, and its appearance is often sharp and vibrant under the right conditions.
Common Copper Compounds
The following are some of the most common copper salts that display this distinctive color when exposed to heat:
- Copper(II) chloride (CuCl₂): Produces a bright green color.
- Copper(II) sulfate (CuSO₄): Generates a blue-green to turquoise shade.
- Copper(II) nitrate (Cu(NO₃)₂): Emits a bluish-green color.
Each of these compounds exhibits a different intensity of the greenish-blue hue, but the characteristic copper color remains consistent across various copper salts. This visual trait is essential for identifying copper in mixtures or experiments where its presence needs to be confirmed quickly and accurately.
Comparing Results with Standards
When conducting high-temperature analyses to identify various elements, it’s essential to compare the observed results with known standards. This comparison allows for accurate identification and helps verify the presence of specific substances based on the unique color emissions they produce. The process is simple yet effective, providing a visual method for confirming the composition of unknown samples.
Standard Colors for Common Elements

Each element, when subjected to heat, emits a characteristic color that can be compared to reference charts for identification. Below is a table displaying some of the most common elements and their associated color emissions when heated:
| Element | Color Emission |
|---|---|
| Sodium | Yellow |
| Potassium | Lilac or Light Purple |
| Calcium | Orange-Red |
| Copper | Green or Blue-Green |
| Strontium | Crimson Red |
By comparing the observed color from a sample to these standards, it is possible to identify the elements present. The consistency of these colors under controlled conditions allows for reliable identification, provided the sample is free from contaminants that could alter the color emissions.
Ensuring Accuracy
While the color comparison method is generally reliable, factors such as the purity of the sample, the temperature of the heat source, and the presence of other substances may influence the results. To ensure accuracy, it is important to use pure samples and maintain consistent heating conditions. Comparing results with a known reference standard is crucial for confirming the identity of elements, particularly in educational or scientific settings where precision is vital.
Common Errors in Flame Test Interpretation
While performing high-temperature analysis to identify elements, several common mistakes can lead to inaccurate interpretations. These errors often arise from misidentification of colors, improper sample preparation, or environmental factors. Understanding these issues is crucial for ensuring the reliability of the results and preventing misleading conclusions.
One of the most frequent mistakes is confusing the color emissions of different elements. Elements like sodium and lithium may emit similar yellowish hues, leading to incorrect identification if the sample is not thoroughly prepared. Another error occurs when the sample is contaminated by other substances, which can alter the emission colors, making it difficult to identify the primary element accurately.
Inaccurate temperature control is another common issue. If the heat source is not consistent, the element may not reach the necessary excitation level, leading to weak or altered color emissions. Likewise, the presence of external lighting or ambient conditions can interfere with color perception, resulting in misidentification.
Finally, inexperienced practitioners might incorrectly compare observed colors with reference charts, particularly if the emission color is faint or distorted. Proper training and careful attention to detail can significantly reduce these errors, improving the accuracy of the analysis.
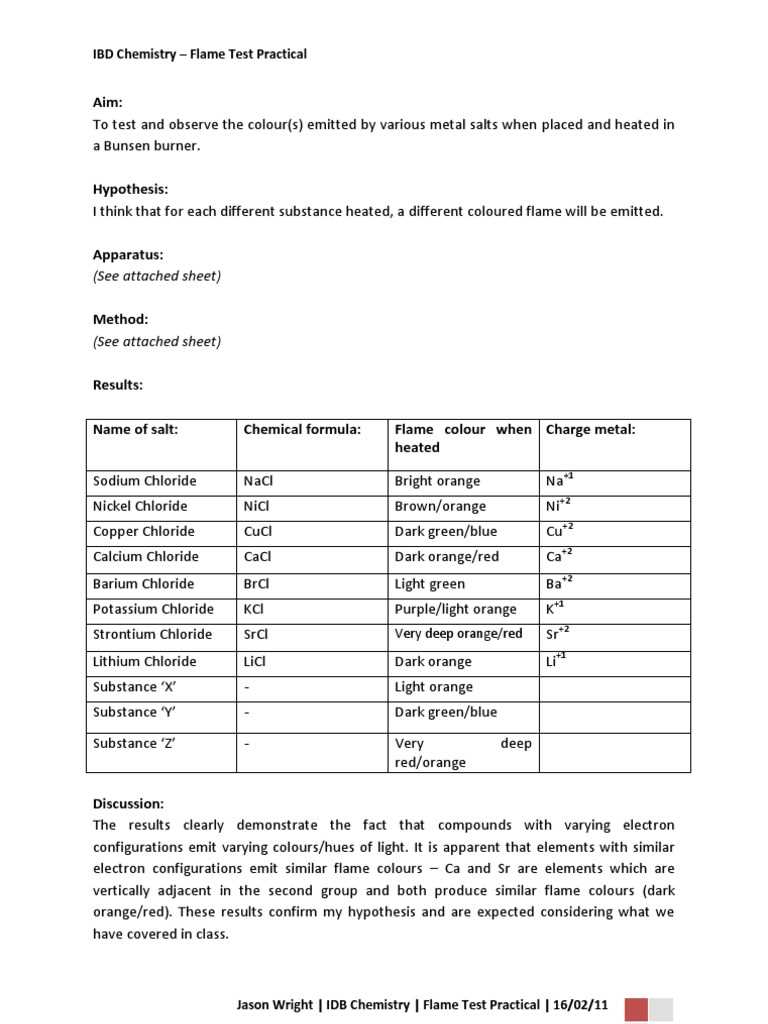
Applications of Flame Test in Chemistry
The high-temperature analysis of elements has a wide range of uses in the field of chemistry. This technique is particularly valuable for identifying metals and certain non-metals by observing their characteristic light emissions when exposed to heat. By studying the emitted colors, scientists can gain insights into the atomic structure and chemical composition of various substances. Below are some key areas where this method is applied:
- Qualitative Analysis: It is often used to identify the presence of specific elements in unknown samples. The unique color each element emits helps in distinguishing between different substances, especially in qualitative analysis.
- Elemental Identification in Compounds: This technique aids in determining the elemental composition of chemical compounds, assisting chemists in verifying the presence of specific metals and their ions in samples.
- Teaching and Education: In educational settings, this method serves as a practical demonstration of how heat energy can excite atoms, providing students with a visual understanding of atomic structure and electron transitions.
- Environmental and Industrial Applications: It is used in industries such as environmental monitoring to detect metal pollutants or in the analysis of minerals and ores in geological studies.
By leveraging this method, chemists are able to quickly and effectively identify elements, making it an essential tool in both research and practical applications.
Flame Test Answer Key and Lab Report Tips
When conducting experiments involving the observation of color changes due to heat exposure, it’s crucial to document results accurately and systematically. A thorough lab report not only helps in understanding the process but also ensures reliable analysis and conclusions. Below are essential tips for creating an effective report and interpreting the outcomes:
- Clear Hypothesis and Objective: Begin your report by stating the purpose of the experiment and the hypothesis you aim to test. Make sure to outline the specific goals and what you expect to observe.
- Accurate Observations: Record the colors produced by each substance carefully. This step is fundamental in identifying the elements based on the visual cues from the emitted light. Be precise and note any variations, as even small differences can indicate a specific element.
- Include Controls: Use a standard reference sample to compare the results. This allows you to verify the consistency and accuracy of your observations.
- Data Presentation: Organize your findings in a clear format, such as tables or charts, for easy comparison. A well-structured presentation helps in understanding the relationship between the element and its emitted color.
- Conclusion and Analysis: Conclude by interpreting the results, comparing them to known standards, and explaining any discrepancies. Address whether the hypothesis was supported by the findings and suggest possible improvements or further investigations.
By following these tips, you can create a comprehensive and accurate report that not only demonstrates your findings but also enhances your understanding of the underlying chemical principles at work.