
In the world of scientific practice, performing controlled experiments is essential to gaining insights into various phenomena. The ability to conduct these experiments accurately and interpret the results is crucial for anyone involved in practical science. This section explores the foundational techniques and problem-solving strategies required to tackle typical experiments and their corresponding challenges.
Mastering the basics of performing scientific procedures involves understanding the methods, equipment, and steps required for precise experimentation. With a solid grasp of these principles, students and practitioners alike can make meaningful discoveries and draw valid conclusions from their findings.
Throughout this guide, we will explore typical issues faced during practical experiments and offer strategies for overcoming them. By learning how to carefully analyze and calculate results, one can enhance both the quality and reliability of their work, making the entire process more efficient and insightful.
Small Scale Chemistry Manual Solutions
Understanding the steps involved in any scientific experiment is crucial for achieving accurate and reliable results. In order to successfully execute a series of practical tasks, it’s important to break down each step, identify common pitfalls, and apply strategies to mitigate errors. This section covers key solutions to typical challenges encountered during experiments, focusing on practical approaches and effective methods.
Effective problem-solving starts with a solid foundation in the fundamental processes. Here are some essential solutions and strategies to ensure success:
- Proper Reagent Preparation: Always ensure reagents are prepared accurately according to specified measurements. This reduces the chance of errors during reactions.
- Accurate Measurements: Use calibrated instruments for precise volume and mass measurements. This minimizes inconsistencies and ensures reproducibility.
- Clear Observation Recording: Document every observation in a structured manner to avoid missing key changes during reactions. Accurate records help in the analysis process.
- Methodical Experimentation: Follow each step of the experiment in a logical order. Skipping steps or improvising can lead to unreliable results.
In addition to these solutions, understanding common mistakes is just as vital. Some common challenges include:
- Incorrect Timing: Some reactions require precise timing. Failing to follow the timing can result in incomplete reactions or unwanted by-products.
- Contamination of Samples: Contaminants can alter the outcome of reactions. Always ensure clean equipment and careful handling of substances.
- Improper Disposal: Waste disposal must be done correctly to avoid contamination and safety hazards. Dispose of materials following the recommended protocols.
By applying these strategies and being mindful of common challenges, one can improve the quality and reliability of their experimental outcomes. Mastering these methods will enhance the overall learning experience and ensure that valuable insights are gained from every experiment.
Understanding Experimental Procedures in Chemistry

Performing experiments requires a structured approach to ensure that each step is carried out accurately and that the results are meaningful. Proper understanding of the process, including the tools and methods involved, is essential for achieving consistent and reliable outcomes. It is important to follow a clear sequence of operations, from preparation to execution, to obtain useful data.
Key factors influencing the success of any experiment include the choice of methods, equipment, and how well the steps are followed. Below is an overview of common procedures and practices:
| Step | Description |
|---|---|
| Preparation | Gathering necessary materials and ensuring they are ready for use. This may include measuring reagents and setting up equipment. |
| Execution | Carefully following the steps outlined in the procedure to carry out the experiment, including mixing substances or applying heat as needed. |
| Observation | Carefully recording any changes or results during the experiment, such as color shifts, gas production, or temperature variations. |
| Analysis | Examining the recorded data to determine patterns, draw conclusions, and identify any discrepancies in expected results. |
| Reporting | Summarizing the methods, results, and conclusions. This is often written in a report or shared in a group discussion to refine understanding. |
By breaking down each phase of an experiment into manageable steps, it becomes easier to identify any issues that may arise and to ensure that accurate data is collected. Mastering these procedures is fundamental to successful experimentation in any scientific field.
Key Techniques in Small Scale Labs
When conducting experiments, mastering fundamental techniques is essential for ensuring accuracy and consistency. These methods form the backbone of reliable scientific inquiry and help in achieving precise results with limited resources. In any practical setting, knowing the right approach for handling materials, controlling variables, and measuring substances can make a significant difference in the outcome of an experiment.
Here are some critical techniques used in controlled environments:
- Precise Measurement: Using calibrated instruments for accurate readings of mass, volume, and temperature is fundamental. Even slight deviations can lead to significant errors in results.
- Proper Mixing: Thorough and consistent mixing ensures reactions proceed efficiently and uniformly. Techniques such as stirring or shaking are essential to achieving uniform results.
- Temperature Control: Maintaining a stable temperature is crucial for many reactions. Devices like water baths or heating plates are often used to regulate and monitor heat during procedures.
- Filtration: When separating substances, using the right filtration method (e.g., gravity or vacuum filtration) is key for obtaining pure samples.
- Volumetric Analysis: Accurate measurement of liquids using graduated cylinders, burettes, or pipettes ensures that solutions are prepared and used at the correct concentrations.
- Observation and Recording: Carefully noting any physical changes, such as color shifts, precipitate formation, or gas evolution, allows for proper analysis and interpretation of results.
These techniques, when executed with precision, help to minimize errors and increase the reliability of experimental findings. Mastering each technique allows for better control over the experiment, leading to more accurate results and a deeper understanding of the underlying principles at work.
Common Challenges in Chemistry Experiments

Even experienced practitioners face obstacles when conducting scientific experiments. Some challenges are intrinsic to the nature of the reactions themselves, while others arise from external factors like equipment or human error. Recognizing and addressing these issues is key to achieving accurate and reproducible results. By understanding common difficulties, one can take proactive measures to minimize mistakes and improve overall performance.
Accuracy in Measurements

One of the most common challenges is achieving precise measurements. Whether it’s determining the exact volume of a liquid or the weight of a substance, even small deviations can lead to significant discrepancies in results. Using improperly calibrated instruments, not following the correct procedures for measurement, or environmental factors like temperature fluctuations can all introduce errors.
Handling Reagents and Materials
Improper handling of reagents and materials can result in contamination, degradation, or loss of substance, all of which affect the outcome of an experiment. Using expired chemicals, inaccurate dilution, or mixing incompatible substances can lead to unexpected results. Furthermore, errors in storage or improper disposal of materials can lead to safety hazards or unreliable data.
By carefully addressing these and other challenges, one can enhance the reliability and reproducibility of their experimental work. Awareness of common issues is the first step in preventing errors and ensuring successful outcomes in future endeavors.
How to Interpret Lab Results Effectively
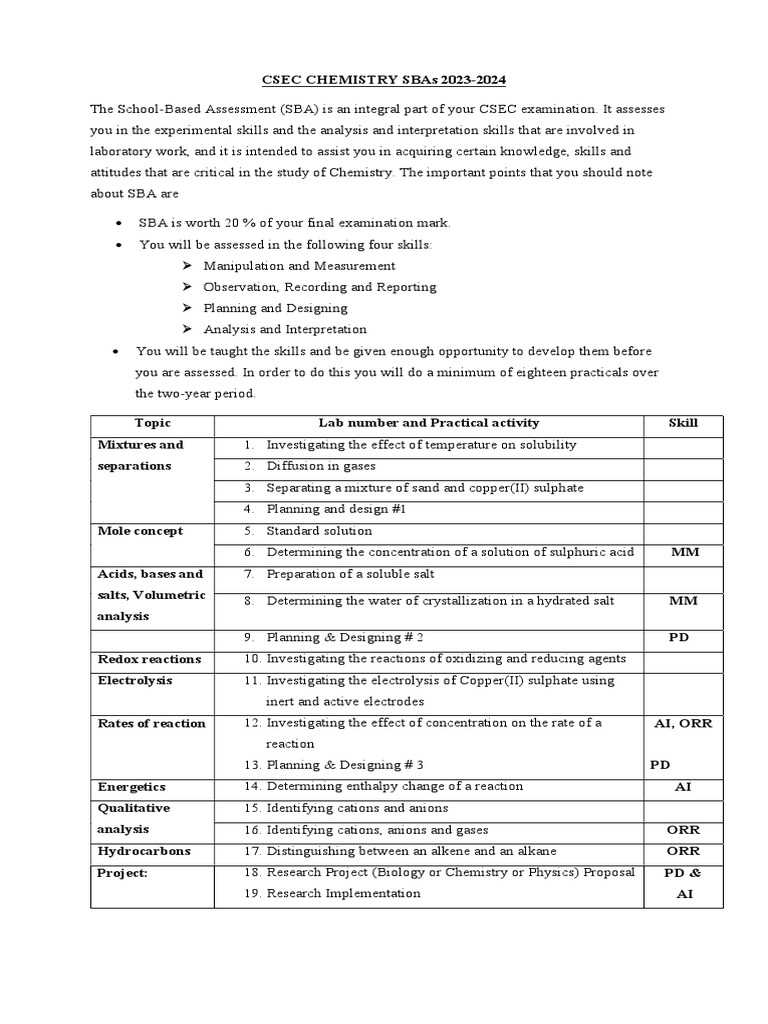
Interpreting results is a critical step in any experimental process, as it allows one to draw meaningful conclusions from the data collected. The ability to accurately analyze the information and identify patterns or trends is essential for understanding the outcomes and making informed decisions. Proper interpretation ensures that the conclusions drawn are valid and based on solid evidence.
Analyzing Data Consistently
The first step in interpreting results is to carefully review the data collected during the experiment. This includes checking for any inconsistencies, such as unexpected values or discrepancies that may suggest errors in the process. It’s important to ensure that the measurements are accurate and that they align with the expected outcomes based on the procedure followed. Using graphs or charts can often help visualize trends and make data easier to analyze.
Comparing with Theoretical Expectations
Once the data has been reviewed, the next step is to compare the results with theoretical predictions or known values. This comparison helps to identify whether the experiment proceeded as expected and whether the results align with established scientific principles. Significant deviations may indicate an issue with the method, reagents, or external factors influencing the experiment. By comparing the observed data to theoretical expectations, one can better understand the reliability of the results.
Accurate interpretation of experimental results is crucial for drawing correct conclusions and advancing scientific knowledge. By applying these strategies, one can ensure that their analysis is both thorough and reliable.
Essential Equipment for Small Scale Labs

Having the right tools is crucial for the success of any scientific procedure. The equipment used in experimental settings directly impacts the precision and reliability of results. While large-scale setups may require specialized instruments, many basic procedures can be carried out effectively with minimal and portable tools. Understanding which instruments are necessary and how to use them properly ensures that the experiment is conducted smoothly and accurately.
Key Instruments for Accurate Measurements
Measurement is one of the most critical aspects of any experiment. Below is a list of essential instruments used to ensure precise readings:
| Instrument | Purpose |
|---|---|
| Graduated Cylinder | Used to measure the volume of liquids with high accuracy. |
| Balance | Essential for weighing substances precisely, ensuring accurate mass measurements. |
| Pipette | Allows for the transfer of small quantities of liquids with high accuracy. |
| Thermometer | Measures the temperature during reactions or processes. |
Basic Equipment for Mixing and Processing
In addition to measuring instruments, mixing and processing tools are necessary for conducting experiments. These tools facilitate the combination of reagents, control over the reaction environment, and separation of components.
| Instrument | Purpose |
|---|---|
| Beaker | A versatile container used for mixing and holding liquids. |
| Stirring Rod | Used for gently stirring solutions to ensure uniformity. |
| Funnel | Assists in transferring liquids or powders into containers with narrow openings. |
| Filter Paper | Used for separating solid particles from liquids during filtration. |
By having the right tools on hand, one can ensure that each step of the process is carried out with precision and that the results are accurate and reproducible. The effectiveness of any experiment is closely tied to the quality of the equipment used, so investing in reliable instruments is essential for achieving reliable outcomes.
Safety Protocols in Chemistry Labs
Ensuring safety during any scientific process is paramount, as handling various substances and equipment can present numerous risks. From toxic chemicals to open flames, the potential for accidents is always present, making it essential to adhere to strict safety procedures. Understanding and following established protocols not only protects individuals but also helps to maintain the integrity of the experiment and prevents the loss of valuable data.
The key to a safe working environment lies in preparation, awareness, and the correct use of protective gear. Every individual in the lab must understand the hazards associated with the materials and equipment they are using, and take necessary precautions to avoid accidents. This includes the use of personal protective equipment (PPE), proper ventilation, and following emergency procedures in case of an accident.
Among the core safety measures, proper labeling, storage of substances, and the knowledge of emergency exits and first-aid kits are fundamental. The following guidelines should always be observed:
- Wear Protective Gear: Always wear safety goggles, gloves, and lab coats to protect against spills, splashes, and airborne particles.
- Know the Risks: Familiarize yourself with the properties of the substances you’re working with and understand their potential hazards.
- Maintain Cleanliness: A tidy workspace minimizes the risk of spills and accidents. Always clean up spills immediately and dispose of waste properly.
- Proper Ventilation: Ensure that the workspace is well-ventilated to avoid exposure to toxic fumes or gases.
- Emergency Procedures: Know the location of emergency exits, fire extinguishers, eye wash stations, and first-aid kits, and be prepared to act quickly in case of an emergency.
By strictly following these protocols, individuals can significantly reduce the risk of accidents and ensure that scientific work is carried out in a safe and controlled manner. A safe working environment fosters productivity, promotes responsible practices, and upholds the safety and well-being of everyone involved.
Preparing Reagents for Small Scale Experiments
Preparing the necessary substances for scientific experiments is a crucial step that requires precision and care. The quality and accuracy of the reagents used directly influence the outcome of the experiment, making it essential to follow proper procedures. Whether mixing solutions, diluting compounds, or preparing solid materials, the correct preparation ensures that the experiment can proceed smoothly and yield reliable results.
One of the primary tasks when working with reagents is ensuring their proper concentration and purity. Incorrect concentrations can lead to invalid results, while impurities might introduce unwanted variables. Careful measurement, proper mixing techniques, and appropriate storage are key factors to ensure reagents are prepared correctly.
Here are the essential steps to prepare reagents accurately:
- Accurate Measurement: Always use calibrated equipment such as pipettes, burettes, and balances to ensure precise measurements of solids and liquids.
- Correct Mixing: When preparing solutions, add solutes to solvents slowly, stirring gently to ensure complete dissolution. Follow the exact steps outlined for each specific reagent.
- Use of Purity Standards: If working with powders or crystals, ensure they are of the highest purity. Impure substances can cause inconsistencies in results.
- Proper Labeling: Clearly label all reagents with their contents, concentration, date of preparation, and any relevant safety information. This helps to avoid errors and ensures safe handling.
- Safe Storage: Store reagents according to their requirements, whether in a cool, dry place or in specific containers to prevent degradation or contamination.
By following these basic principles, scientists and researchers can prepare reagents effectively, ensuring that their experiments are based on the most reliable and accurate materials available. Proper reagent preparation is a fundamental skill in any scientific field, crucial for obtaining meaningful and reproducible results.
Analyzing Chemical Reactions and Observations
Understanding and interpreting reactions are essential for drawing meaningful conclusions from any experiment. Whether it’s a color change, the formation of a precipitate, or the release of gas, observing these physical and chemical changes is crucial for understanding the behavior of substances under specific conditions. Properly analyzing these changes helps to confirm hypotheses and provides insight into the underlying processes driving the transformation of materials.
The process of analyzing reactions involves a combination of qualitative and quantitative assessments. Qualitative analysis focuses on identifying the substances involved based on observable changes, while quantitative analysis seeks to measure the extent of the reaction and determine the amounts of substances used or produced. Accurate observations and proper documentation of these changes are vital to the reliability of the results.
Key factors to consider when analyzing reactions include:
- Color Changes: Many reactions cause distinct color changes, which can indicate the formation of new compounds or the completion of a reaction. Carefully noting these shifts helps to identify the substances involved.
- Formation of Precipitates: The appearance of solid particles in a liquid solution is a strong indicator of a chemical reaction. Observing when and how these particles form can offer clues about the reaction mechanism.
- Gas Evolution: The release of gases during a reaction, often seen as bubbling or effervescence, can point to the production of a volatile compound. Identifying the gas produced is essential for understanding the reaction type.
- Temperature Changes: An increase or decrease in temperature during a reaction suggests whether it is exothermic or endothermic, providing valuable information about energy exchanges within the system.
- Time and Rate: Monitoring the speed at which a reaction progresses can help determine the reaction rate, which is influenced by factors such as concentration, temperature, and the presence of catalysts.
By carefully documenting and analyzing these observations, one can gain a deeper understanding of the reactions taking place and draw conclusions that contribute to broader scientific knowledge. Reactions often offer more than just answers–they provide insight into the interactions and dynamics of matter, helping to refine experimental techniques and guide future investigations.
Understanding Chemical Equations and Balancing
Chemical equations represent the transformations that occur during a reaction. They provide a symbolic way to describe how reactants are converted into products, showing the relationship between the substances involved. The process of balancing these equations is essential to ensure that the law of conservation of mass is followed–meaning that matter is neither created nor destroyed during a reaction. Achieving balance requires adjusting the coefficients of each substance in the equation, ensuring that the number of atoms of each element is the same on both sides.
The Basics of Chemical Equations
A chemical equation consists of two main parts: the reactants and the products. Reactants are the substances that undergo change during the reaction, while products are the new substances formed. These substances are connected by an arrow, indicating the direction of the transformation. A basic equation looks like this:
| Reactants | Arrow (→) | Products |
|---|---|---|
| 2H₂ + O₂ | → | 2H₂O |
In this example, hydrogen and oxygen are the reactants, and water is the product. Notice that the equation is not yet balanced; the number of hydrogen and oxygen atoms differs on both sides.
Balancing the Equation
To balance the equation, we adjust the coefficients (the numbers placed in front of each substance) so that the number of atoms of each element is equal on both sides of the equation. This is done by ensuring that for every atom of an element in the reactants, there is an equal number in the products. In the example above, the balanced equation would look like this:
| Reactants | Arrow (→) | Products |
|---|---|---|
| 2H₂ + O₂ | → | 2H₂O |
In this case, there are two hydrogen molecules and one oxygen molecule on the left side, and two water molecules on the right side, ensuring that the number of atoms is equal for both hydrogen and oxygen.
Balancing chemical equations is a fundamental skill in science, providing clarity about the quantities of substances involved in a reaction. By following systematic methods and practicing with different reactions, one can gain proficiency in interpreting and balancing equations, which is critical for understanding the stoichiometry of chemical processes.
Tips for Accurate Measurement in Labs

Accurate measurement is a cornerstone of successful scientific experimentation. Precise data is crucial for obtaining reliable results and drawing valid conclusions. Whether you’re working with liquids, solids, or gases, the ability to measure quantities with precision can significantly affect the outcome of your experiments. This section provides essential tips for ensuring accurate measurements in any setting.
Essential Tools for Precision
Using the right tools is key to achieving accurate measurements. Different instruments are designed for different tasks, and selecting the appropriate one can make all the difference. Here are some common tools and their recommended uses:
- Balances: Use high-quality balances for measuring mass. Always calibrate the balance before use to ensure accurate readings.
- Graduated Cylinders: These are ideal for measuring liquid volumes. Always check the meniscus (the curve at the surface of the liquid) at eye level to avoid parallax error.
- Pipettes: For small liquid measurements, use pipettes or burettes, which offer a higher degree of precision than larger containers.
- Thermometers: Ensure that the thermometer is calibrated and that it is used within its specified range to maintain accuracy when measuring temperature.
Best Practices for Accurate Measurements
Accuracy also depends on how measurements are taken. Here are some best practices to follow:
- Always Calibrate Equipment: Before using any measuring device, ensure it is properly calibrated. A small error in calibration can result in significant inaccuracies.
- Avoid Contamination: Clean all measuring instruments thoroughly before use. Even trace amounts of previous substances can affect the measurements.
- Measure at the Right Temperature: Temperature fluctuations can affect the volume of liquids and gases. Measure substances at the correct temperature or adjust for thermal expansion or contraction if necessary.
- Use Consistent Techniques: Apply consistent techniques for every measurement. Whether it’s the way you read a meniscus or the angle at which you hold a thermometer, consistency ensures precision.
- Take Multiple Measurements: For more accurate results, take multiple measurements and calculate the average. This helps minimize human error and anomalies in data.
By following these simple yet effective guidelines, you’ll be able to achieve more reliable and consistent results, which is crucial for making accurate conclusions and advancing scientific understanding.
Common Errors in Laboratory Experiments
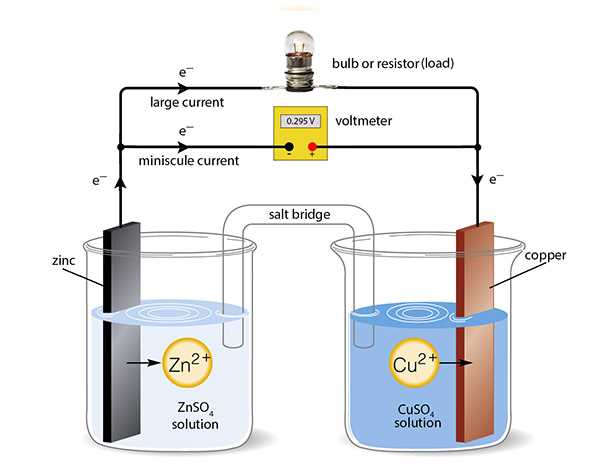
Even experienced individuals can encounter challenges while conducting experiments. Mistakes are common, and recognizing them is an essential step in ensuring reliable results. Identifying and addressing errors early on can prevent inaccurate conclusions and improve the overall quality of scientific work. In this section, we’ll discuss some frequent mistakes made during experimental procedures and how to avoid them.
Measurement and Calculation Mistakes

Errors in measurement and calculations can lead to significant discrepancies in experimental outcomes. Here are some of the most common errors related to these areas:
- Incorrect Calibration: Using improperly calibrated instruments can introduce systematic errors, affecting the accuracy of the results. Always ensure that balances, pipettes, and other measuring devices are calibrated properly before use.
- Reading Meniscus Incorrectly: When measuring liquids in a graduated cylinder, reading the meniscus from an incorrect angle can lead to false volume readings. Always ensure the line of sight is at eye level.
- Failure to Convert Units: Failing to convert between units when needed can result in calculation errors, especially when working with different measurement systems.
- Inconsistent Measurements: Inaccurate results can arise if measurements are not taken consistently, such as using varying techniques or tools for different trials.
Experimental Procedure Mistakes
Beyond measurement errors, mistakes in following procedures can also lead to incorrect results. Here are some procedural pitfalls to watch out for:
- Inaccurate Timing: Many experiments require precise timing. Starting or stopping a timer too early or too late can result in invalid data, especially in reactions or rate-based experiments.
- Contaminated Samples: Cross-contamination from previous experiments or improper handling of materials can affect the purity of samples, leading to skewed results.
- Improper Handling of Reagents: Using incorrect quantities or improperly mixing substances can alter the expected outcomes. Always follow the guidelines for reagent handling carefully.
- Environmental Factors: Variables like temperature, humidity, or air pressure can interfere with experimental conditions. Failing to control these factors may lead to inaccurate or inconsistent results.
By recognizing and addressing these common errors, you can minimize mistakes and improve the reliability of your experimental results. Awareness and attention to detail are critical components of successful scientific investigations.
How to Document Lab Results Properly
Proper documentation is crucial for ensuring that experimental data is both accurate and useful for future reference. Without clear and organized records, it can be difficult to reproduce results, analyze trends, or draw meaningful conclusions. Effective documentation provides a detailed account of all procedures, observations, and results, allowing for better collaboration and more reliable scientific work. This section will explore essential techniques for documenting experimental outcomes in a way that is clear, consistent, and comprehensive.
When recording results, it is important to maintain consistency and clarity throughout the process. Start by noting the basic details of the experiment, such as the objectives, materials used, and methods followed. Ensure that all measurements are precise and that the conditions under which the experiment was conducted are clearly stated. This helps others understand how the data was obtained and makes it easier to identify any sources of error.
Additionally, it’s important to include observations in a systematic way. Whether the results align with expectations or not, documenting the observations thoroughly can provide insights into potential sources of discrepancies. Use clear units and appropriate significant figures to avoid ambiguity.
Following a structured format for recording data can also improve the organization of results. This might include:
- Tables: Present data in organized tables, where measurements and observations can be easily compared.
- Charts/Graphs: Visual representation of data can help identify trends and patterns.
- Notes on Anomalies: Document any unusual occurrences, unexpected outcomes, or deviations from expected results.
- Conclusions: Summarize the key findings, including whether the hypothesis was supported or not, and suggest possible reasons for any discrepancies.
By following these guidelines and being diligent in recording every detail, you ensure that your results are useful, reproducible, and accurate. Proper documentation not only helps you track your own progress but also aids others who may want to verify or build upon your work.
Approaching Laboratory Questions Methodically
When tackling questions related to experiments, a structured approach is essential for accurate and reliable responses. Rather than rushing to conclusions, it’s important to take the time to think critically, break down the problem, and address each aspect systematically. By following a clear method, you can ensure that your answers are based on solid reasoning and evidence, leading to more meaningful results and interpretations.
To approach experimental questions effectively, consider the following steps:
- Understand the Question: Before starting, make sure you fully comprehend the question. Identify what is being asked and determine the key concepts or processes involved.
- Review Relevant Concepts: Refresh your understanding of the scientific principles or theories that relate to the experiment. This can help clarify the expected outcomes and guide your interpretation of the data.
- Analyze the Data: Look carefully at the experimental results. Are there any noticeable trends or patterns? Compare your observations with your expectations or theoretical predictions.
- Formulate a Hypothesis: Based on the data and your understanding of the concepts, try to form a hypothesis or explanation. This should directly address the question at hand, using the information available.
- Verify with Evidence: Cross-check your hypothesis with the available experimental evidence. Ensure that your conclusion aligns with the results and is backed by the data.
- Consider Alternatives: If the data doesn’t fully support your initial hypothesis, think of alternative explanations or possibilities that could explain the outcomes. This helps you build a well-rounded understanding of the results.
By taking this methodical approach, you increase the likelihood of providing accurate, well-reasoned answers to any experimental question. Not only does this lead to a deeper understanding of the material, but it also ensures that your findings are reliable and scientifically sound.
Calculations in Laboratory Work
In scientific experiments, accurate calculations are crucial for interpreting results, determining quantities, and ensuring precision. Whether you’re preparing solutions, calculating concentrations, or determining yields, precise mathematical operations are essential for obtaining reliable and reproducible data. A strong understanding of the underlying principles and proper calculation techniques can significantly enhance the quality of experimental work.
Key aspects of calculations in experimental procedures include:
- Concentration Determination: Knowing how to calculate concentrations is fundamental in many experiments. This includes working with molarity, normality, or parts per million, depending on the required precision and context.
- Stoichiometric Calculations: In reactions, stoichiometry helps determine the amounts of reactants and products involved. By applying the molar ratios derived from balanced equations, you can calculate the necessary quantities to achieve desired outcomes.
- Yield Calculations: Yield calculations are vital to evaluate the efficiency of a reaction. Comparing theoretical yields to actual yields helps assess the effectiveness of an experiment and identify any potential losses or errors.
- Unit Conversion: Often, data in experiments need to be converted from one unit to another. This requires familiarity with conversion factors and ensuring the correct application of units in each calculation.
- Significant Figures: When performing calculations, it’s important to maintain proper significant figures. This ensures that your results reflect the precision of your measurements and are consistent with the experimental limits.
By mastering these essential calculation techniques, you can ensure that your results are accurate and meaningful, supporting reliable conclusions and further research. Always double-check your work to avoid errors and maintain the integrity of your findings.
Improving Lab Efficiency and Accuracy
Maximizing both speed and precision is crucial when conducting scientific experiments. Achieving efficiency without sacrificing accuracy can significantly enhance the quality and reliability of your results. Streamlining processes, minimizing errors, and optimizing the use of resources are key strategies that can lead to more successful and consistent experimental outcomes. Whether it’s through better preparation, equipment usage, or attention to detail, there are several ways to improve your overall performance in the lab.
Key strategies for improving efficiency and accuracy include:
- Thorough Preparation: Prior to starting an experiment, ensure that all materials, tools, and chemicals are ready and available. This prevents delays during the procedure and reduces the likelihood of mistakes caused by searching for missing items.
- Optimizing Workflow: Organize the workspace to minimize distractions and unnecessary movements. A well-organized bench allows for quicker access to instruments and reagents, improving the overall pace of the experiment.
- Using Proper Measurement Techniques: Accurate measurements are essential for reliable results. Employing calibrated instruments and using correct methods for volume, mass, and temperature measurements will prevent inaccuracies and errors.
- Minimizing Contamination: Contamination can lead to unreliable data and flawed conclusions. Ensuring that equipment is clean, chemicals are pure, and handling procedures are correct can prevent such issues.
- Careful Data Recording: Maintaining precise and detailed records throughout the experiment can help track any discrepancies, errors, or unexpected results. This will also make it easier to identify patterns and ensure consistency in future trials.
- Time Management: Effective time management can reduce rush-induced mistakes. Breaking experiments into smaller, manageable steps and allowing adequate time for each task ensures better control over the procedure and the resulting data.
By integrating these techniques into your experimental routine, you can achieve more reliable outcomes, reduce variability, and increase your productivity. Improved efficiency and accuracy lead to more dependable data and ultimately greater success in scientific research.