
Achieving success in scientific assessments requires a deep understanding of key concepts, critical thinking, and effective study techniques. Whether you’re preparing for a high-stakes test or simply seeking to improve your knowledge, having a solid approach is crucial. The ability to apply theory to practical problems and recall essential information under pressure is what distinguishes top performers.
In this guide, we will explore various strategies to help you tackle complex problems with confidence. By focusing on important principles, mastering problem-solving techniques, and organizing your study materials, you can enhance both your knowledge and test-taking abilities. Each topic will provide useful insights to refine your skills and boost your overall performance.
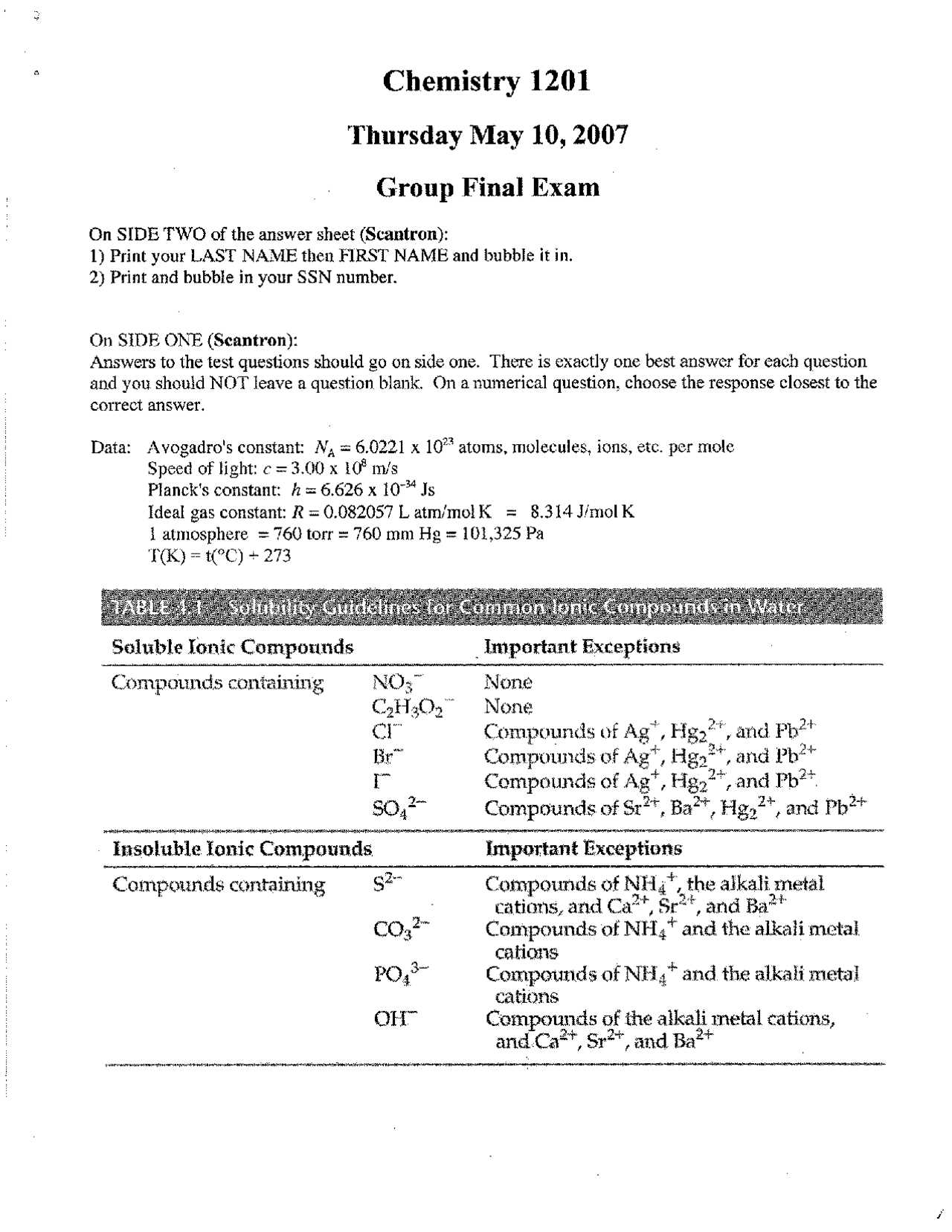
Final Exam Chemistry Answers
To excel in science assessments, it’s crucial to focus on both theoretical knowledge and practical problem-solving skills. Mastering the core principles and being able to apply them effectively under test conditions can make a significant difference. Preparation involves not only understanding the material but also practicing techniques to navigate complex questions efficiently.
Here are some key strategies for success:
- Understand Core Principles: Focus on grasping the fundamental concepts behind each topic. Knowing why a process occurs will help you answer questions more confidently.
- Practice Problem-Solving: Solve as many sample problems as possible. This will help you recognize patterns and approaches needed to tackle different types of questions.
- Use Study Aids: Flashcards, summary notes, and online resources can reinforce key points and provide a quick review.
- Review Mistakes: Review practice tests and assignments to understand where you went wrong. Correcting errors early will improve accuracy.
- Time Management: Allocate specific time blocks for different sections during the test. Practice answering questions within the allotted time to simulate test conditions.
Additionally, knowing how to approach certain types of questions, such as those involving calculations or interpreting data, is essential for success. By applying these strategies, you will not only improve your knowledge but also your ability to perform under pressure.
Key Concepts for Chemistry Success
Mastering the essential principles of science is fundamental for achieving success in assessments. A solid understanding of core topics provides the foundation for solving complex problems and applying concepts in various scenarios. Without a strong grasp of basic ideas, even the most advanced questions can become challenging.
Focus on the following key areas to build a strong knowledge base:
- Atomic Structure and Bonding: Understanding the arrangement of atoms, electron configurations, and how atoms bond is crucial for solving a wide range of problems.
- Stoichiometry: Learn how to balance equations and calculate reactant and product quantities accurately.
- Thermodynamics: Grasping the principles of energy, heat, and work is essential for interpreting various chemical reactions.
- Kinetics: Study how reaction rates are affected by temperature, concentration, and catalysts.
- Acid-Base Equilibria: Familiarize yourself with pH calculations, neutralization reactions, and buffer solutions.
Focusing on these core areas will provide a comprehensive understanding that is necessary for tackling both theoretical and practical questions. A deep knowledge of these topics will help you answer questions with confidence and efficiency, ensuring better performance in any evaluation.
Understanding Chemical Reactions for Exams
Grasping the dynamics of how substances interact and transform is a cornerstone of science assessments. The ability to recognize different types of reactions and predict their outcomes plays a vital role in solving related problems. A thorough understanding of reaction mechanisms, balancing equations, and the factors influencing reaction rates can significantly enhance your performance.
To better understand reactions, focus on these key elements:
- Types of Reactions: Familiarize yourself with the common types of reactions such as synthesis, decomposition, combustion, and displacement. Knowing these will help you identify reaction patterns quickly.
- Balancing Equations: Practice balancing chemical equations. This skill is crucial for understanding stoichiometry and determining the proportions of reactants and products.
- Reaction Rates: Study how temperature, concentration, and catalysts affect the speed of reactions. Understanding these variables will help you answer related questions effectively.
- Le Chatelier’s Principle: Learn how systems at equilibrium respond to changes in concentration, pressure, and temperature. This principle is often tested in reaction-related problems.
- Redox Reactions: Understand oxidation and reduction processes, as well as how to identify electron transfer during reactions.
By focusing on these core aspects of chemical reactions, you’ll be able to approach related questions with confidence. Practicing these principles will not only improve your understanding but also enhance your ability to apply them in various test scenarios.
Important Formulas to Memorize
Knowing essential mathematical relationships is critical for solving many scientific problems efficiently. Formulas serve as tools that help you understand the underlying principles and carry out calculations with precision. Memorizing key equations ensures you can quickly apply them when needed, saving valuable time during assessments.
Below are some of the most important formulas to keep in mind:
| Formula | Description |
|---|---|
| PV = nRT | Ideal gas law, relating pressure, volume, temperature, and number of moles of gas |
| c = λν | Relationship between the speed of light (c), wavelength (λ), and frequency (ν) |
| q = mcΔT | Formula for heat transfer, where q is heat, m is mass, c is specific heat, and ΔT is temperature change |
| E = mc² | Energy-mass equivalence from Einstein’s theory of relativity |
| n = m/M | Calculation for number of moles (n), mass (m), and molar mass (M) |
These formulas are foundational for solving a wide variety of problems. Regular practice and understanding their applications will improve both your efficiency and accuracy in answering questions during assessments.
How to Tackle Organic Chemistry Questions

Approaching questions related to molecular structures, reactions, and mechanisms requires a solid understanding of the principles behind organic compounds. These types of problems often involve complex reasoning, and a systematic approach can make them much more manageable. By focusing on patterns, functional groups, and reaction mechanisms, you can improve your ability to analyze and solve organic-related problems effectively.
Here are some tips for tackling these types of questions:
- Know the Functional Groups: Memorize the different functional groups and their reactivity. Recognizing these groups quickly will help you understand how molecules behave in various reactions.
- Practice Reaction Mechanisms: Understanding the step-by-step processes of reactions will allow you to predict the products and understand how intermediates form. Focus on common mechanisms like substitution, elimination, and addition.
- Draw Structures: Visualizing molecules and reaction pathways by drawing structures can help you see relationships and clarify complex problems. This is especially useful for stereochemistry and reaction intermediates.
- Understand Stereochemistry: Pay attention to the three-dimensional arrangement of atoms. Know how to determine chirality, enantiomers, and diastereomers, as these are frequently tested in organic-related problems.
- Memorize Key Reagents: Certain reagents are crucial for specific reactions. Familiarize yourself with common reagents and their functions in various organic transformations.
By honing these skills and practicing regularly, you’ll become more adept at tackling challenging organic-related questions, allowing you to solve them with greater efficiency and confidence.
Mastering the Periodic Table for Exams
Understanding the organization and patterns within the periodic table is essential for solving many scientific problems. The table not only provides information about the elements’ properties but also offers insights into their behavior during reactions. By recognizing trends in atomic size, electronegativity, ionization energy, and other characteristics, you can predict how elements will interact in various scenarios.
Key Trends to Focus On
Pay attention to the periodic trends that govern element behavior. These trends are essential for answering a variety of questions efficiently:
- Atomic Size: Learn how atomic radius changes across periods and groups. Elements in the same group tend to have larger atomic radii as you move down, while they decrease across a period from left to right.
- Electronegativity: The tendency of an atom to attract electrons increases across a period and decreases down a group. This is particularly useful when dealing with bonding and reactivity questions.
- Ionization Energy: Understand how ionization energy increases across periods and decreases down groups, as it affects how easily an element can form ions.
Strategies for Memorizing Elements
To efficiently recall the elements and their properties, use the following techniques:
- Mnemonics: Create mnemonic devices to help remember the order of elements, especially for groups like alkali metals, halogens, and noble gases.
- Practice with Flashcards: Use flashcards to test your knowledge of element names, symbols, and atomic numbers.
- Group Elements by Similarities: Focus on the characteristics that elements in the same group share. This helps in remembering properties such as reactivity and bonding behavior.
Mastering these aspects of the periodic table will greatly enhance your ability to answer questions related to element properties and behavior in reactions, making it an invaluable tool during assessments.
Balancing Chemical Equations Quickly
Balancing equations is a crucial skill in solving problems related to chemical reactions. The process ensures that the law of conservation of mass is followed, meaning the same number of atoms of each element are present on both sides of the equation. Developing an efficient method for balancing equations can save time and help improve accuracy, especially under test conditions.
Step-by-Step Approach
Start by following a systematic approach to balance equations effectively:
- Write the Unbalanced Equation: Begin by writing the chemical formula for all reactants and products without worrying about the balance.
- Balance Atoms One Element at a Time: Focus on balancing the elements that appear the least. Adjust the coefficients to ensure the same number of atoms on both sides for each element.
- Balance Oxygen and Hydrogen Last: These elements often appear in multiple compounds, so it’s best to adjust them after all other elements are balanced.
- Check the Balance: After adjusting coefficients, review the equation to ensure that each element is balanced. Verify that all coefficients are in the smallest whole number ratio.
Tips for Speeding Up the Process
Here are some additional strategies to help you balance equations quickly:
- Use Fractions if Necessary: If balancing with whole numbers is difficult, start with fractions and multiply through to eliminate them.
- Look for Common Patterns: Certain types of reactions, like combustion or acid-base reactions, follow predictable patterns. Recognizing these can save time.
- Practice Regularly: The more equations you balance, the quicker and more efficient you’ll become at spotting patterns and making adjustments.
By practicing these techniques and focusing on a systematic approach, you’ll be able to balance chemical equations quickly and accurately, improving both your speed and confidence in problem-solving scenarios.
Preparing for Stoichiometry Problems
Succeeding in problems involving the calculation of reactants and products requires a solid understanding of relationships between different substances in a chemical reaction. These problems often involve using mole ratios, converting between units, and applying the law of conservation of mass. By mastering these calculations, you can approach stoichiometry problems with confidence and accuracy.
Understanding the Basics

To tackle stoichiometry challenges, it’s important to first review the basic concepts:
- Molar Mass: Know how to calculate the molar mass of compounds. This is key for converting between grams and moles, which is often the first step in solving stoichiometry problems.
- Mole Ratios: Understand how to use coefficients from the balanced chemical equation to set up mole ratios, which allow you to relate different substances in the reaction.
- Conversion Factors: Be familiar with various conversion factors, such as converting grams to moles, moles to particles, or liters to moles for gases.
Effective Strategies for Success
When preparing for problems involving stoichiometry, consider the following strategies:
- Practice Unit Conversions: Regularly practice converting between grams, moles, liters, and molecules. This will help you become more efficient when solving stoichiometric problems.
- Work with Balanced Equations: Ensure that you always start with a properly balanced equation. This step is essential for determining accurate mole ratios and performing correct calculations.
- Double-Check Calculations: Always verify your results to ensure that all units cancel appropriately and that your final answer makes sense in the context of the problem.
By understanding these key concepts and practicing regularly, you’ll be well-prepared to solve stoichiometry problems quickly and effectively, leading to greater success in assessments.
Common Mistakes to Avoid in Chemistry
In the study of chemical reactions and processes, students often make a few key mistakes that can lead to incorrect conclusions or answers. Recognizing and avoiding these common errors is essential for improving problem-solving skills and achieving accuracy in calculations. Being aware of these pitfalls can help enhance understanding and prevent confusion during assessments.
Some of the most frequent mistakes include:
- Neglecting to Balance Equations: Failing to balance chemical equations before proceeding with calculations is a common oversight that can lead to incorrect results. Always ensure that the number of atoms is equal on both sides.
- Misunderstanding Unit Conversions: Incorrectly converting units, such as grams to moles or liters to molecules, is a frequent issue. Mastering these conversions is crucial for accurate calculations.
- Overlooking Significant Figures: Ignoring the rules for significant figures can lead to precision errors in your answers. Always ensure that your final result reflects the correct number of significant digits.
- Forgetting to Check Units: Leaving units unchecked or failing to cancel them out properly can result in answers that do not make sense. Always track your units throughout the problem-solving process.
- Confusing Reaction Mechanisms: Failing to correctly identify or understand the reaction mechanism can cause errors in predicting products or reactant behavior. A solid grasp of common reaction pathways is essential.
By staying mindful of these mistakes and practicing regularly, you can avoid pitfalls and improve both your speed and accuracy in solving chemical problems.
Effective Time Management During Tests
Time management is a crucial skill when taking any assessment, especially in subjects that require complex problem-solving. Properly allocating time for each question and avoiding spending too much time on one problem can make the difference between completing the test successfully and running out of time. Effective planning allows you to approach each section with confidence and ensure that you maximize your performance.
Strategies for Efficient Time Allocation
Start by dividing the test into sections, and allocate time based on the number of questions or the difficulty of the content:
- Estimate Time per Section: Before you begin, quickly assess how much time you should spend on each part of the test. For example, if there are 50 questions and 90 minutes, aim to spend about 1-2 minutes per question, but adjust for more complex problems.
- Prioritize Easy Questions: Begin with questions that you find easiest or are most confident in. This will help build momentum and secure quick points before tackling more difficult problems.
- Mark and Move On: If you come across a question you’re stuck on, mark it and move on to the next. Returning to tough questions later with a fresh mind often leads to better solutions.
Avoiding Time-Wasting Mistakes

Being aware of common time-wasting habits can help you stay focused and manage your time more effectively:
- Don’t Overthink Questions: Once you’ve answered a question, don’t dwell on it. Double-checking is important, but excessive rethinking can waste valuable time.
- Stay Calm Under Pressure: If you start feeling stressed, take a few deep breaths. Staying calm will help you think more clearly and avoid rushing through questions.
By practicing time management strategies and staying organized, you’ll be able to tackle any assessment with greater efficiency, ensuring that you can answer every question and avoid unnecessary pressure.
How to Approach Multiple Choice Questions

Multiple choice questions are a common format in assessments, requiring careful thought and strategy. They test your ability to recall information quickly and apply it accurately. While they may seem straightforward, a systematic approach can help you improve your chances of selecting the correct answer, even when you’re uncertain about the specifics.
Steps to Maximize Your Performance
To increase your efficiency when answering multiple choice questions, consider the following tips:
- Read the Question Carefully: Start by reading the question and all answer choices thoroughly before making a decision. Pay attention to key terms, and make sure you understand what is being asked.
- Eliminate Clearly Wrong Answers: If you’re unsure of the correct answer, eliminate the choices that are clearly incorrect. This increases your odds of selecting the correct one from the remaining options.
- Look for Clues in the Question: Often, the question itself can provide hints about the correct answer. Pay attention to words like “always,” “never,” or “most likely” as they can indicate the most accurate choice.
Handling Difficult Questions
When faced with tough questions, don’t panic. Instead, try these strategies:
- Take an Educated Guess: If you’re not sure, rely on your general knowledge and intuition to make an educated guess. Avoid leaving questions blank if you have no time to return to them later.
- Review Your Answer: Once you’ve selected an answer, quickly review the question and options again. Sometimes, you may notice a mistake or find a better choice upon re-evaluating.
By staying focused, managing your time wisely, and using these strategies, you can approach multiple choice questions with greater confidence and increase your likelihood of success.
Essential Lab Skills for Chemistry Exams
Practical laboratory skills are an integral part of any scientific assessment. The ability to perform experiments accurately, follow procedures precisely, and interpret results is essential for achieving success. Mastering these skills not only helps during hands-on assessments but also strengthens your understanding of theoretical concepts. Knowing how to efficiently work in the lab will boost your overall performance and help you apply theoretical knowledge to real-world situations.
Key Laboratory Techniques to Master
There are several essential skills that every student should be familiar with to navigate lab-based tasks effectively:
- Proper Measurement and Calibration: Accurately measuring liquids and solids is fundamental. Be comfortable using pipettes, burettes, and balances, and always calibrate your equipment before starting any experiment.
- Handling Chemicals Safely: Knowing how to handle, store, and dispose of chemicals properly is crucial. Always wear protective gear and be aware of the safety procedures in case of spills or accidents.
- Observing and Recording Data: Precision in recording data is essential for drawing accurate conclusions. Ensure all observations, measurements, and notes are clear, concise, and organized.
Strategies for Effective Experimentation
In addition to technical skills, developing a thoughtful approach to laboratory work will increase your efficiency:
- Plan Your Experiments: Before starting any procedure, review the steps, identify the equipment you’ll need, and familiarize yourself with the expected outcomes.
- Stay Organized: A well-organized workspace and systematic approach to conducting experiments will prevent errors and save time.
- Review Results Carefully: Once the experiment is complete, analyze your results and check for consistency. Repetition may be necessary to ensure accuracy.
By refining these skills and practicing them regularly, you can confidently approach any lab task and significantly enhance your performance in assessments that involve practical components.
Tips for Memorizing Chemical Structures
Understanding and recalling molecular structures is essential for many scientific assessments. Mastering these visual representations allows you to predict properties, reactions, and behavior of substances. While memorization can be challenging, employing effective strategies can significantly improve retention and recall. By breaking down the process into manageable steps, you can enhance your ability to recognize and reproduce complex structures with confidence.
Effective Techniques for Retention
To successfully memorize molecular structures, consider these methods:
- Practice Regularly: The more you draw and visualize structures, the easier it becomes to remember them. Create flashcards with different molecules and their corresponding structures to reinforce memory.
- Break Down the Structure: Focus on understanding the basic components–atoms, bonds, and functional groups–before trying to memorize the entire molecule. Recognizing patterns and common groupings can simplify the process.
- Use Mnemonics: Develop simple mnemonic devices to associate structures with familiar images or words. This can help trigger recall when you need it most.
Visualization and Application
In addition to rote memorization, applying visual techniques can strengthen your memory:
- Draw the Structures: Repetition through drawing allows your brain to physically engage with the structure, reinforcing the mental image.
- Teach What You Learn: Explaining structures to someone else helps you reinforce your understanding and memory. Teaching forces you to organize your knowledge in a way that’s easier to recall.
With consistent practice and the right strategies, memorizing chemical structures becomes more manageable. The key is to stay patient, use diverse techniques, and engage with the material actively to ensure long-term retention.
Preparing for Acid-Base Titrations
Acid-base titrations are essential techniques used to determine the concentration of an unknown solution by neutralizing it with a solution of known concentration. This process requires precision, patience, and a clear understanding of the underlying concepts, including the selection of appropriate indicators and the accurate measurement of volumes. To perform titrations successfully, it is vital to be well-prepared and familiar with both the theory and the practical steps involved.
Key Steps in Acid-Base Titration
Proper preparation and a systematic approach are crucial for carrying out a successful titration. Below are the essential steps:
- Prepare the Solutions: Ensure that both the acid and base solutions are accurately prepared and their concentrations are known. This is important for calculating the results.
- Select the Right Indicator: Choose an appropriate pH indicator that changes color at the equivalence point of the titration. Common indicators include phenolphthalein and methyl orange.
- Set Up the Equipment: Properly calibrate the burette and pipette. Ensure all glassware is clean and free of contaminants to avoid errors in measurement.
Understanding the Titration Process
During the titration, one solution is gradually added to the other until the equivalence point is reached. The key to accuracy is ensuring that you can detect the point at which neutralization occurs. Below is a table outlining the titration process:
| Step | Description |
|---|---|
| 1. Add the known solution | Fill the burette with the solution of known concentration (titrant), and place the unknown solution in the flask. |
| 2. Add indicator | Introduce a few drops of the chosen pH indicator into the unknown solution to help identify the endpoint. |
| 3. Titrate | Slowly add the titrant to the unknown solution while stirring, watching for the color change indicating neutralization. |
| 4. Calculate the result | Use the volume of the titrant and its concentration to calculate the concentration of the unknown solution. |
By following these steps carefully and practicing regularly, you can ensure that your titration results are both accurate and reliable. Understanding the theory behind the technique and mastering the practical skills involved will prepare you for successful titrations in both laboratory and assessment settings.
How to Solve Thermodynamics Problems
Thermodynamics is a fundamental area of study that explores the relationships between heat, energy, and work within a system. Solving thermodynamics problems involves understanding key principles, such as energy conservation, the laws of thermodynamics, and the behavior of gases and other substances under various conditions. Whether you’re dealing with heat transfer, enthalpy changes, or calculating efficiency, a systematic approach is essential for success.
To solve these types of problems effectively, it is important to follow a series of logical steps. First, identify the known and unknown variables, then select the relevant equations and principles that apply to the problem. Pay close attention to the conditions provided, such as temperature, pressure, and volume, as these factors can significantly influence the calculations. Lastly, ensure that units are properly converted and consistent throughout the process.
Here are some key concepts to consider when tackling thermodynamics questions:
- First Law of Thermodynamics: Energy cannot be created or destroyed, only transferred or converted from one form to another. Use this principle to relate work, heat, and internal energy.
- Enthalpy Changes: Changes in enthalpy (ΔH) help calculate heat exchange at constant pressure. Understanding how to calculate this is crucial for solving many thermodynamic problems.
- Ideal Gas Law: For problems involving gases, use the ideal gas law (PV = nRT) to relate pressure, volume, temperature, and moles of gas.
- Entropy: Entropy measures the disorder or randomness of a system. Understanding entropy changes helps solve problems related to reversible and irreversible processes.
By breaking down each problem into smaller, manageable steps and applying these core concepts, you can navigate thermodynamics questions with confidence. With practice, you will improve both your problem-solving speed and accuracy, leading to better understanding and results in this challenging area of study.
Reviewing Past Exam Papers for Success
One of the most effective ways to prepare for upcoming assessments is to review previous test papers. This strategy helps you understand the types of questions that are commonly asked, the level of detail required in your responses, and how to manage your time during the actual test. By analyzing past papers, you can identify patterns and focus on areas where you may need additional practice or improvement.
Start by collecting as many past papers as you can. These are often available through your instructor, online resources, or your school’s archives. Once you have them, follow these steps to maximize your study session:
- Understand the Question Format: Review the structure of different question types (multiple choice, short answer, problem-solving) to familiarize yourself with what to expect.
- Identify Key Topics: Highlight recurring themes and topics. Focus on areas that appear consistently across various papers, as these are likely to be important for future assessments.
- Practice Under Time Constraints: Simulate the test environment by setting a time limit. This will help improve your time management skills and increase your efficiency when answering questions.
- Analyze Your Mistakes: After completing a past paper, go over the answers carefully. Understand why you got certain questions wrong and revisit those topics to reinforce your knowledge.
- Improve Your Writing Skills: In addition to solving problems, practice writing clear and concise answers. Pay attention to how your responses are structured and whether you are providing enough detail where necessary.
By regularly reviewing past papers and focusing on these key strategies, you will not only improve your knowledge but also enhance your ability to tackle various types of questions with confidence. This targeted approach to studying can significantly boost your performance when it matters most.