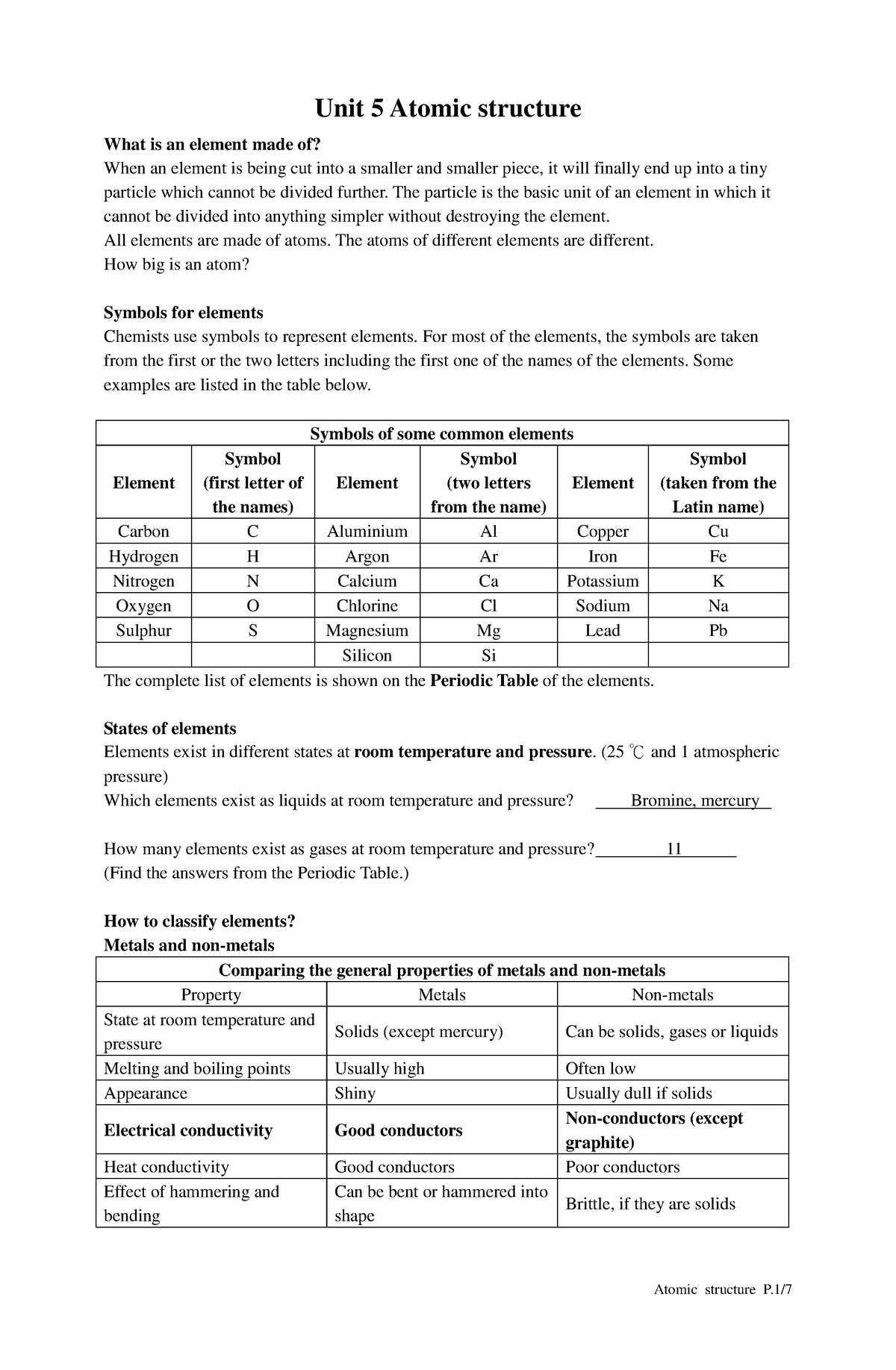
In this section, we will explore some of the most important principles and techniques crucial for understanding advanced scientific topics. Whether you’re preparing for exams or simply looking to strengthen your grasp of the material, this guide will help clarify complex concepts and provide clear explanations.
Each section focuses on different areas of study, breaking down essential formulas, methods, and calculations to ensure a solid foundation. With focused practice and a deeper understanding of the core ideas, you’ll be well-equipped to tackle problems efficiently and accurately.
As you progress, you’ll discover how to approach common challenges, avoid common errors, and apply your knowledge to solve real-world problems. Through detailed examples and effective strategies, you’ll build confidence in your abilities and enhance your overall comprehension.
Overview of Key Concepts in the Section
This section provides a comprehensive outline of the critical principles and techniques essential for mastering the topics covered. The focus is on understanding fundamental ideas and applying them to various types of problems. Key concepts will be examined in detail to enhance both theoretical knowledge and practical skills.
Throughout this guide, emphasis is placed on:
- Understanding the core principles and how they relate to real-world scenarios.
- Mastering essential formulas and calculations.
- Identifying common challenges and developing strategies to overcome them.
By the end of this section, you will be able to approach complex questions with confidence, making connections between theoretical knowledge and its practical applications. Each concept is broken down to ensure clarity and ease of understanding, with a focus on building a strong foundation for further learning.
Key Concepts in the Section

In this part of the material, we delve into the fundamental ideas that form the basis of the subject. Understanding these concepts is essential for mastering the more advanced topics. The focus is on building a clear foundation that will support further learning and problem-solving.
Some of the most important concepts to grasp include:
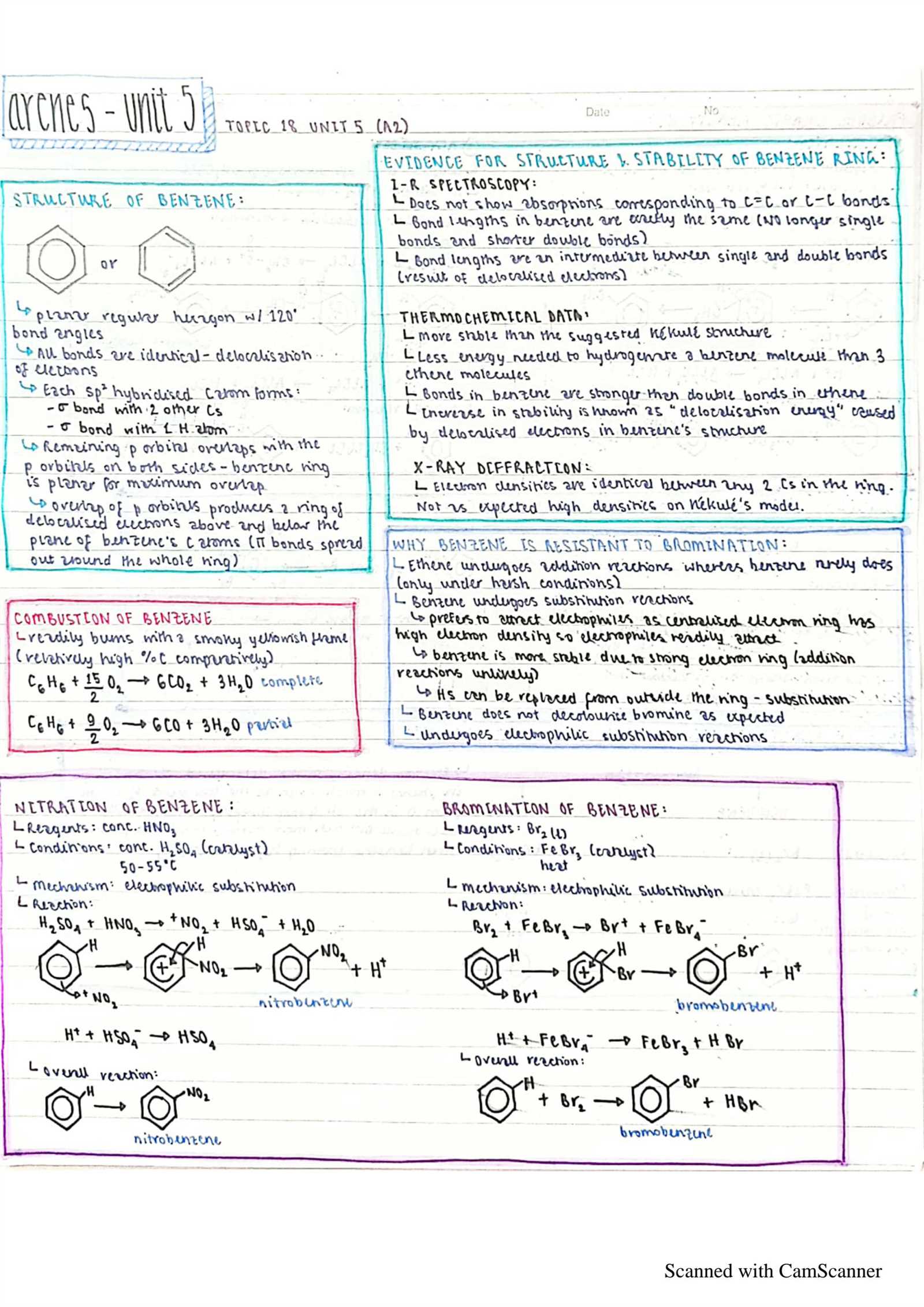
- Atomic Structure: Understanding the arrangement of subatomic particles and how they influence chemical behavior.
- Bonding: Recognizing the different types of bonds (ionic, covalent) and how atoms combine to form molecules.
- Stoichiometry: Learning how to use ratios and proportions to solve quantitative problems involving chemical reactions.
- Thermodynamics: Analyzing energy changes during chemical reactions and understanding key terms like enthalpy and entropy.
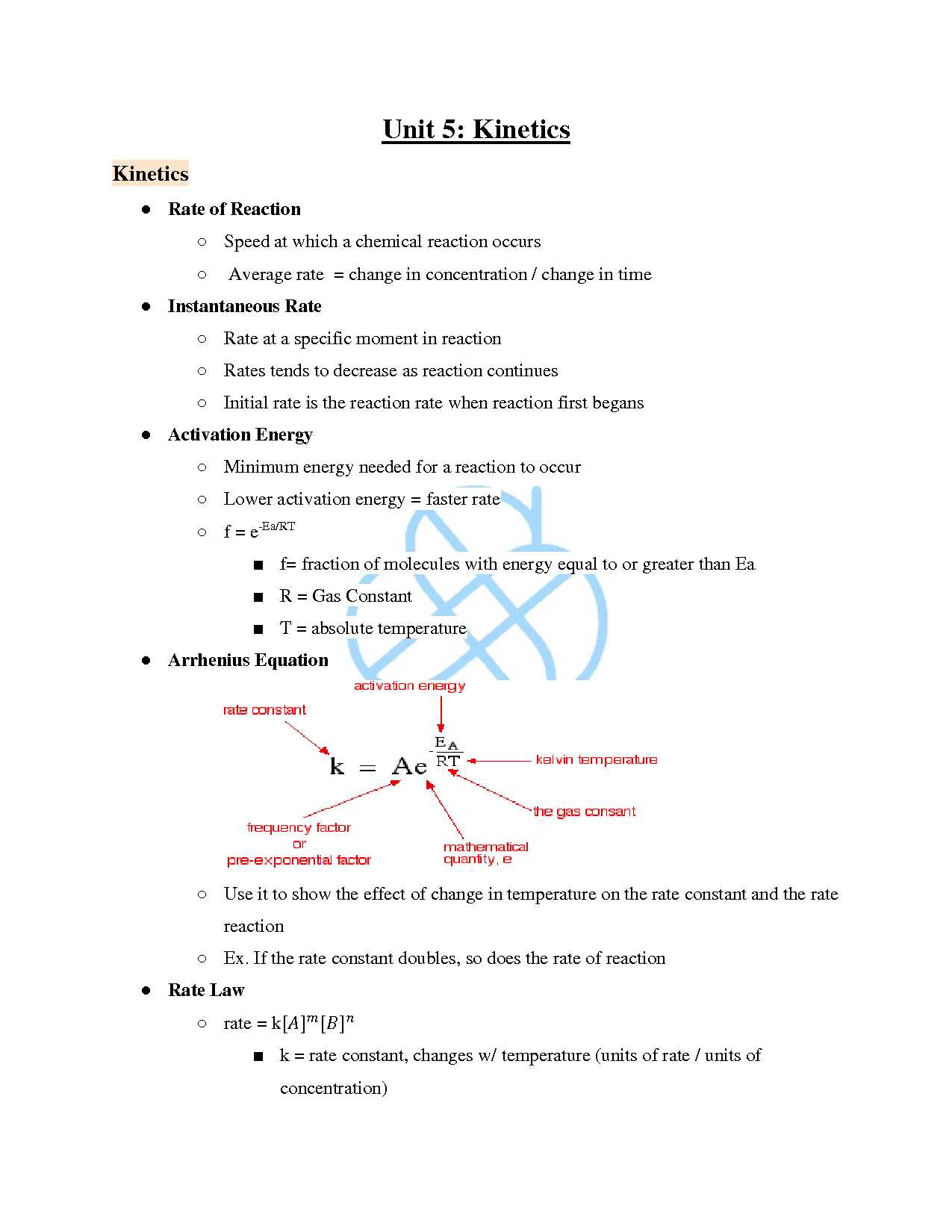
- Kinetics: Studying the factors that affect the rate of reactions and how to calculate reaction speeds.
Mastering these core ideas will provide a strong framework for tackling problems and applying theoretical knowledge to practical situations. As you progress, these concepts will allow you to approach more complex topics with greater ease and understanding.
Important Formulas to Remember
Formulas play a crucial role in solving problems and understanding the relationships between different variables. They are essential tools for making accurate calculations and applying theoretical knowledge to practical situations. Below are some of the most vital formulas that should be committed to memory for success in this section.
- Ideal Gas Law: PV = nRT
– This formula relates pressure, volume, temperature, and the amount of substance in a gas. - Molarity: M = n/V
– Used to calculate the concentration of a solution, where n is the number of moles and V is the volume of the solution. - Density: ρ = m/V
– The formula for density, where ρ is density, m is mass, and V is volume. - Stoichiometry Ratio: moles of A / moles of B
– Used to relate the amount of one substance to another in a chemical reaction. - Enthalpy Change: ΔH = H(products) – H(reactants)
– This calculates the heat change during a reaction at constant pressure.
These formulas are fundamental tools that allow you to solve various types of problems efficiently. Understanding their derivation and application will greatly enhance your ability to work through complex questions with ease.
Common Mistakes in the Section

As with any challenging subject, it’s easy to make errors that can lead to confusion or incorrect results. Understanding these common mistakes can help you avoid them and improve your problem-solving approach. Below are some of the most frequently made errors in this area of study.
Calculation Errors
- Forgetting to convert units before applying formulas.
- Mixing up significant figures and rounding prematurely.
- Incorrectly balancing equations, leading to wrong stoichiometric ratios.
- Not properly accounting for temperature or pressure changes in gas calculations.
Conceptual Misunderstandings
- Confusing different types of bonds (ionic vs. covalent) and their properties.
- Misapplying the ideal gas law when the conditions do not meet the assumptions.
- Forgetting to account for limiting reactants in chemical reactions.
- Overlooking the role of catalysts in reaction rates.
By being aware of these mistakes, you can take proactive steps to prevent them. Double-checking calculations, thoroughly understanding concepts, and practicing a variety of problems will help you avoid these common pitfalls and enhance your mastery of the material.
Tips for Effective Study Sessions
Maximizing the effectiveness of your study time is essential for mastering complex subjects. By following a few strategic approaches, you can improve your focus, retain information better, and increase your overall efficiency. Here are some practical tips to enhance your study sessions and make them more productive.
Time Management Strategies
- Set clear, achievable goals for each session to stay focused on specific topics.
- Use the Pomodoro technique: study for 25 minutes, followed by a 5-minute break to maintain concentration.
- Prioritize difficult topics when your energy levels are highest, typically at the beginning of a study session.
- Track your progress with a checklist to stay motivated and see tangible results.
Active Learning Techniques
- Teach what you’ve learned to someone else; explaining concepts reinforces your understanding.
- Practice with problem sets and quizzes to apply knowledge in various scenarios.
- Summarize information in your own words to reinforce retention.
- Use visual aids, like diagrams and charts, to better understand complex relationships and processes.
By incorporating these strategies into your study routine, you can ensure that each session is both productive and effective, leading to improved performance and deeper comprehension.
Understanding Chemical Reactions
Chemical reactions are fundamental processes that transform substances into new products. Recognizing how and why reactions occur is essential for solving problems and predicting outcomes. This section explores the key factors that influence reactions, including the role of energy, reactants, and products, as well as the various types of reactions encountered in this field.
The Basics of Reaction Mechanisms
- Reactions involve the breaking and forming of bonds between atoms.
- The Law of Conservation of Mass states that mass is conserved during a reaction.
- Energy changes, either as heat or light, often accompany reactions, depending on whether they are exothermic or endothermic.
- The rate of a reaction is influenced by factors such as temperature, concentration, and catalysts.
Types of Chemical Reactions
- Synthesis: Two or more reactants combine to form a single product.
- Decomposition: A single compound breaks down into two or more simpler substances.
- Single Replacement: One element replaces another in a compound.
- Double Replacement: Two compounds exchange components to form two new compounds.
- Combustion: A substance reacts with oxygen, often producing energy in the form of heat and light.
By understanding these fundamental principles and the different reaction types, you can predict the products of reactions and better understand the mechanisms driving these transformations. Mastery of these concepts is key to tackling more complex problems and applications.
Balancing Equations Simplified
Balancing equations is a fundamental skill in understanding how substances react with each other. It ensures that the same number of atoms of each element are present on both sides of the equation, adhering to the Law of Conservation of Mass. While it can initially seem challenging, following a systematic approach makes the process straightforward and manageable.
To balance equations effectively, follow these steps:
- Write the unbalanced equation: Start by noting the chemical formulas of the reactants and products.
- Count the atoms: List the number of atoms of each element on both sides of the equation.
- Adjust the coefficients: Begin by balancing the atoms of elements that appear in only one reactant and one product. Use whole number coefficients.
- Balance hydrogen and oxygen last: These elements often appear in multiple compounds, so it’s easier to balance them after other elements are accounted for.
- Check the balance: Verify that the number of atoms for each element is the same on both sides of the equation.
For example, in the reaction of hydrogen and oxygen to form water:
- Unbalanced equation: H2 + O2 → H2O
- Balance the equation: 2H2 + O2 → 2H2O
By practicing these steps, balancing equations becomes a more intuitive process, helping you to approach chemical reactions with greater confidence and accuracy.
Stoichiometry Explained in Detail
Stoichiometry is a critical concept that allows us to calculate the quantities of reactants and products involved in chemical reactions. By understanding the relationship between the amounts of substances, we can predict the outcome of reactions and solve for unknown quantities. This section will break down stoichiometry in detail, providing the tools needed to solve complex problems with ease.
The Basics of Stoichiometric Calculations
At the heart of stoichiometry lies the concept of molar ratios, which relate the quantities of different substances in a reaction. These ratios are derived from the balanced chemical equation and allow us to convert between moles of reactants and products.
- Identify the known quantity: Begin with the amount of a given substance (typically in moles or grams).
- Use the mole ratio: Extract the mole ratio from the balanced equation to relate the quantities of the substances involved.
- Convert units as needed: If the given quantity is in grams, use the molar mass to convert to moles.
- Calculate the unknown quantity: Apply the ratio to find the desired amount of the other substance.
Example Problem
For the reaction:
- 2H2 + O2 → 2H2O
If you start with 4 moles of hydrogen (H2), you can calculate the required amount of oxygen (O2) as follows:
- Use the mole ratio: 2 moles of H2 react with 1 mole of O2.
- 4 moles of H2 would need 2 moles of O2.
By following these steps, you can easily solve stoichiometry problems and determine the quantities of materials involved in any reaction.
Limiting Reactants and Excess Reactants
In many chemical reactions, the reactants are not present in equal amounts, which can affect how much product is produced. The concept of limiting and excess reactants helps us understand which substances are consumed first and which remain after the reaction is complete. Identifying the limiting reactant is crucial for determining the maximum amount of product that can be formed.
The limiting reactant is the one that will run out first, limiting the amount of product that can be produced. On the other hand, the excess reactant is the substance that is left over after the reaction has finished.
How to Identify the Limiting Reactant
- Start with a balanced chemical equation.
- Convert the amount of each reactant to moles.
- Use the mole ratio from the balanced equation to determine how much of each reactant is required to react with the other.
- The reactant that will be used up first (based on the mole ratio) is the limiting reactant.
- The reactant that is left over is the excess reactant.
Example of Limiting and Excess Reactants
Consider the following reaction:
| Reactants | Mole Ratio | Given Amount (moles) | Required Amount (moles) |
|---|---|---|---|
| H2 | 2 | 4 | 4 |
| O2 | 1 | 3 | 2 |
In this example, 4 moles of H2 will react with 2 moles of O2. Since only 3 moles of O2 are available, oxygen is the limiting reactant, and hydrogen is the excess reactant.
By understanding the roles of limiting and excess reactants, you can predict how much product will be produced and how much of each reactant will remain after the reaction is complete.
Review of Molarity Calculations
Molarity is a measure of concentration that expresses the number of moles of a solute in one liter of solution. This concept is essential for understanding how substances dissolve and interact in solutions. In this section, we will revisit the basic steps involved in calculating molarity and applying it to various problems.
The formula for molarity is:
M = n / V
Where:
- M is the molarity (in moles per liter, mol/L).
- n is the number of moles of solute.
- V is the volume of the solution in liters.
To calculate the molarity of a solution, you simply divide the number of moles of the solute by the total volume of the solution in liters. Here’s a step-by-step approach:
- Determine the amount of solute (in moles). This can be found using the molar mass of the substance if the mass is given.
- Measure or calculate the volume of the solution in liters.
- Apply the formula M = n / V to find the molarity.
For example, if you dissolve 0.5 moles of sodium chloride (NaCl) in 2 liters of water, the molarity would be:
M = 0.5 moles / 2 L = 0.25 mol/L
Understanding molarity is crucial when preparing solutions for experiments, calculating concentrations in reactions, and diluting solutions to desired concentrations. With practice, this calculation becomes a fundamental skill for working with solutions in a variety of scientific contexts.
Thermodynamics and Energy Changes

The study of energy and its transformations is central to understanding how reactions occur and why certain processes are spontaneous. Energy changes are an essential aspect of reactions, affecting everything from reaction rates to the final products. In this section, we will explore the concepts of energy transfer, heat, and work, and their impact on chemical processes.
Types of Energy Changes
- Exothermic reactions: These reactions release energy, usually in the form of heat. The products have less energy than the reactants.
- Endothermic reactions: In contrast, endothermic reactions absorb energy from their surroundings, leading to a decrease in temperature around the system. The products have more energy than the reactants.
- Work and Heat: Energy can be transferred to or from a system in the form of work or heat. These transfers play a key role in determining whether a reaction is spontaneous or not.
Understanding Enthalpy
- Enthalpy change (ΔH): This term refers to the heat absorbed or released under constant pressure. It helps determine whether a reaction is exothermic or endothermic.
- Standard enthalpy of formation: The change in enthalpy when one mole of a compound is formed from its elements in their standard states.
- Hess’s Law: The total enthalpy change of a reaction is the sum of the enthalpy changes of the individual steps in the reaction, regardless of the pathway taken.
By understanding these concepts, you can better predict the behavior of systems in terms of energy transfer and determine the conditions under which reactions will take place. This knowledge is crucial in applications ranging from industrial processes to environmental chemistry.
Electrochemistry Key Concepts
Electrochemistry explores the relationship between electricity and chemical reactions. This field involves the study of how electrical energy can drive chemical processes and how chemical reactions can produce electrical energy. Understanding these principles is essential for a variety of applications, from batteries and fuel cells to electroplating and corrosion prevention.
Redox Reactions
- Oxidation: The process in which a substance loses electrons, resulting in an increase in oxidation state.
- Reduction: The process where a substance gains electrons, leading to a decrease in oxidation state.
- Oxidizing and Reducing Agents: The oxidizing agent gains electrons and is reduced, while the reducing agent loses electrons and is oxidized.
- Half-Reactions: Redox reactions are split into two half-reactions–one for oxidation and one for reduction. These half-reactions are crucial for understanding how energy is transferred in electrochemical processes.
Electrochemical Cells
- Galvanic Cells: These are spontaneous electrochemical cells that convert chemical energy into electrical energy. A common example is a battery.
- Electrolytic Cells: These cells require an external power source to drive a non-spontaneous reaction. An example is the process of electroplating, where metal ions are reduced onto a surface.
- Cell Potential: The potential difference between the two electrodes in an electrochemical cell. This is measured in volts and determines the cell’s ability to drive an electric current.
By understanding redox reactions and the functioning of electrochemical cells, we can apply these principles in fields such as energy storage, environmental protection, and material science.
Practice Problems for Mastery
One of the best ways to solidify your understanding of complex concepts is through practice. By tackling a variety of problems, you can apply theoretical knowledge to real-world scenarios, reinforcing your skills and boosting your confidence. This section provides a series of practice problems designed to challenge you and help you master key topics.
Each problem is intended to help you practice critical thinking and problem-solving techniques, whether you’re dealing with stoichiometric calculations, balancing reactions, or understanding energy changes. Be sure to follow the steps carefully, and double-check your work to ensure accuracy. If necessary, break down the problems into smaller parts to understand the underlying concepts better.
Once you feel comfortable with these practice questions, you will be better prepared to tackle more advanced topics and apply your knowledge to new situations.
Problem 1: Molarity Calculation
Calculate the molarity of a solution if 0.75 moles of NaCl are dissolved in 3 liters of water.
Problem 2: Limiting Reactant
In a reaction where 5 moles of hydrogen gas react with 3 moles of oxygen gas, determine the limiting reactant and the excess reactant.
Problem 3: Balancing Reactions
Balance the following chemical equation:
C4H10 + O2 → CO2 + H2O
Problem 4: Thermodynamics
Given the enthalpy change (ΔH) for a reaction is -150 kJ, determine whether the reaction is exothermic or endothermic.
Problem 5: Electrochemical Cell
Describe the function of the anode and cathode in an electrochemical cell and explain their roles in a galvanic reaction.
By practicing these types of problems regularly, you will develop a strong grasp of the material and enhance your ability to solve related questions efficiently during tests or in practical applications.
Using the Periodic Table in Unit 5
The periodic table is a vital tool for understanding the properties and behaviors of elements. In this section, we will explore how the table can be utilized to solve problems and deepen your understanding of various topics. By referring to the table, you can gain insights into atomic structure, electron configurations, and trends that influence reactions and compounds.
Each element on the periodic table is organized according to its atomic number and chemical properties, making it easier to predict how elements will interact in chemical reactions. It also provides key information, such as atomic mass, which is essential when performing calculations related to moles, molarity, and stoichiometry.
Key Features of the Periodic Table
| Feature | Description |
|---|---|
| Atomic Number | The number of protons in an atom, determining the element’s identity and position in the table. |
| Atomic Mass | The weighted average mass of an element’s isotopes, crucial for calculating molar mass in stoichiometry. |
| Groups | Vertical columns that indicate elements with similar chemical properties and the same number of valence electrons. |
| Periods | Horizontal rows that show elements with increasing atomic number and energy levels. |
By understanding these key features, you can use the periodic table to predict trends such as electronegativity, ionization energy, and atomic radius. For instance, knowing the position of an element allows you to anticipate whether it will readily lose or gain electrons, which is important for understanding reaction mechanisms and bonding behavior.
In addition, the periodic table helps in the calculation of molar masses, which are essential for determining the quantities of reactants and products in reactions. Familiarity with the table also aids in solving problems involving stoichiometry, limiting reactants, and balancing chemical equations.
Commonly Asked Exam Questions
Preparing for exams often involves focusing on recurring topics and question types. By practicing questions that are commonly asked, you can sharpen your ability to solve problems efficiently and ensure that you are well-prepared for the most frequently tested concepts. In this section, we will highlight some of the most typical questions you may encounter, as well as provide strategies for answering them effectively.
These questions cover a wide range of topics, from basic calculations to more complex theoretical concepts. Understanding the key principles behind these questions will allow you to tackle them with confidence, while also deepening your grasp of the material. Below are examples of commonly asked questions that can help you prepare for your exam.
Example Exam Questions
| Question Type | Example Question | Key Focus |
|---|---|---|
| Calculation | What is the molarity of a solution containing 2 moles of NaCl dissolved in 4 liters of water? | Molarity, concentration calculations |
| Reaction Mechanism | Balance the following chemical reaction: H2 + O2 → H2O | Balancing chemical equations, stoichiometry |
| Energy Change | Is the following reaction exothermic or endothermic? ΔH = -200 kJ | Understanding thermodynamics, heat of reaction |
| Electrochemistry | What is the role of the anode and cathode in a galvanic cell? | Electrochemical cells, oxidation and reduction |
By practicing these types of questions, you will become familiar with the typical format of exam problems and learn how to apply your knowledge effectively. Focus on understanding the concepts behind each question, as this will help you tackle variations of the same problems with ease.
Additionally, always double-check your work and make sure to follow the correct steps when solving problems, especially when calculations are involved. Time management during the exam is key, so practicing these questions will help you complete each section with greater speed and accuracy.
How to Avoid Test Anxiety
Test anxiety is a common experience that can impact performance, causing stress and self-doubt before and during exams. It can hinder your ability to recall information or think clearly under pressure. However, there are effective strategies to manage anxiety and improve focus, allowing you to perform at your best. In this section, we will explore practical methods to reduce stress and approach your exams with a calm, confident mindset.
Understanding that feeling nervous is normal can help you reframe anxiety as a sign of readiness rather than fear. By implementing techniques such as preparation, relaxation exercises, and time management, you can minimize the impact of anxiety and build a positive attitude towards assessments. The key is to focus on the process rather than the outcome, maintaining a sense of control and resilience.
Effective Strategies to Manage Anxiety
- Preparation: Consistent study and practice lead to confidence. Start early and break down the material into manageable sections to avoid last-minute cramming.
- Visualization: Before the test, take a few minutes to visualize yourself calmly answering questions. Positive imagery can help reduce nervousness and increase self-assurance.
- Relaxation Techniques: Deep breathing, mindfulness, or meditation can help lower stress levels. Incorporate these practices before and during the exam to maintain focus and composure.
- Time Management: During the exam, allocate time wisely. Answer the questions you know first, and leave the more challenging ones for later. This reduces the feeling of being overwhelmed.
- Physical Activity: Regular exercise, even a short walk, helps reduce stress and boosts brain function, enhancing overall performance.
- Sleep and Nutrition: Prioritize rest and balanced meals before the exam. Fatigue and poor nutrition can exacerbate anxiety and decrease focus.
By applying these strategies, you can reduce stress and cultivate a more positive, calm approach to your exams. Remember, test-taking is a skill that improves with practice and mindfulness. Stay focused on your efforts, and trust in your preparation.
Reviewing Key Definitions and Terms
Understanding the fundamental concepts and terminology is essential for mastering any subject. In this section, we will go over some of the most important terms and definitions that are critical for problem-solving and concept application. A strong grasp of these key terms will enable you to approach complex topics with clarity and confidence, as well as improve your ability to answer questions accurately.
Many topics build upon these foundational ideas, so reviewing the key terms regularly will not only help you during exams but also enhance your overall comprehension. Definitions are not just words–they are the building blocks for understanding broader concepts and their applications in various scenarios.
Important Terms to Remember
- Atomic Number: The number of protons in an atom’s nucleus, which defines the element’s identity and determines its position on the periodic table.
- Mol: A unit used to measure the amount of substance. One mole represents approximately 6.022 x 10²³ entities, such as atoms or molecules.
- Concentration: The amount of a substance present in a certain volume of solution, typically expressed in molarity (moles per liter).
- Stoichiometry: The calculation of reactants and products in chemical reactions based on the conservation of mass and the balancing of chemical equations.
- Exothermic Reaction: A reaction that releases heat energy, causing the surrounding environment to become warmer.
- Endothermic Reaction: A reaction that absorbs heat energy from its surroundings, leading to a decrease in temperature around the reaction site.
Additional Key Definitions
- Limiting Reactant: The reactant that is completely consumed in a reaction and determines the maximum amount of product that can be formed.
- Excess Reactant: The reactant that remains after the reaction is complete because it is not fully used up.
- Activation Energy: The minimum energy required for a chemical reaction to occur, often referred to as the “energy barrier” for reactions.
- Electrolyte: A substance that conducts electricity when dissolved in water, usually due to the presence of ions in the solution.
- Redox Reaction: A type of chemical reaction involving the transfer of electrons between substances, leading to changes in oxidation states.
Regularly revisiting and internalizing these definitions will provide a solid foundation for solving problems, writing explanations, and making connections across different topics. Ensure that you can not only define each term but also apply it to various scenarios to strengthen your understanding.