
Preparing for your first major test can be overwhelming, but with the right strategies, you can approach it with confidence. This section offers key insights into tackling the most crucial topics covered during the first part of your course. Understanding the core concepts and knowing how to apply them will make all the difference in your performance.
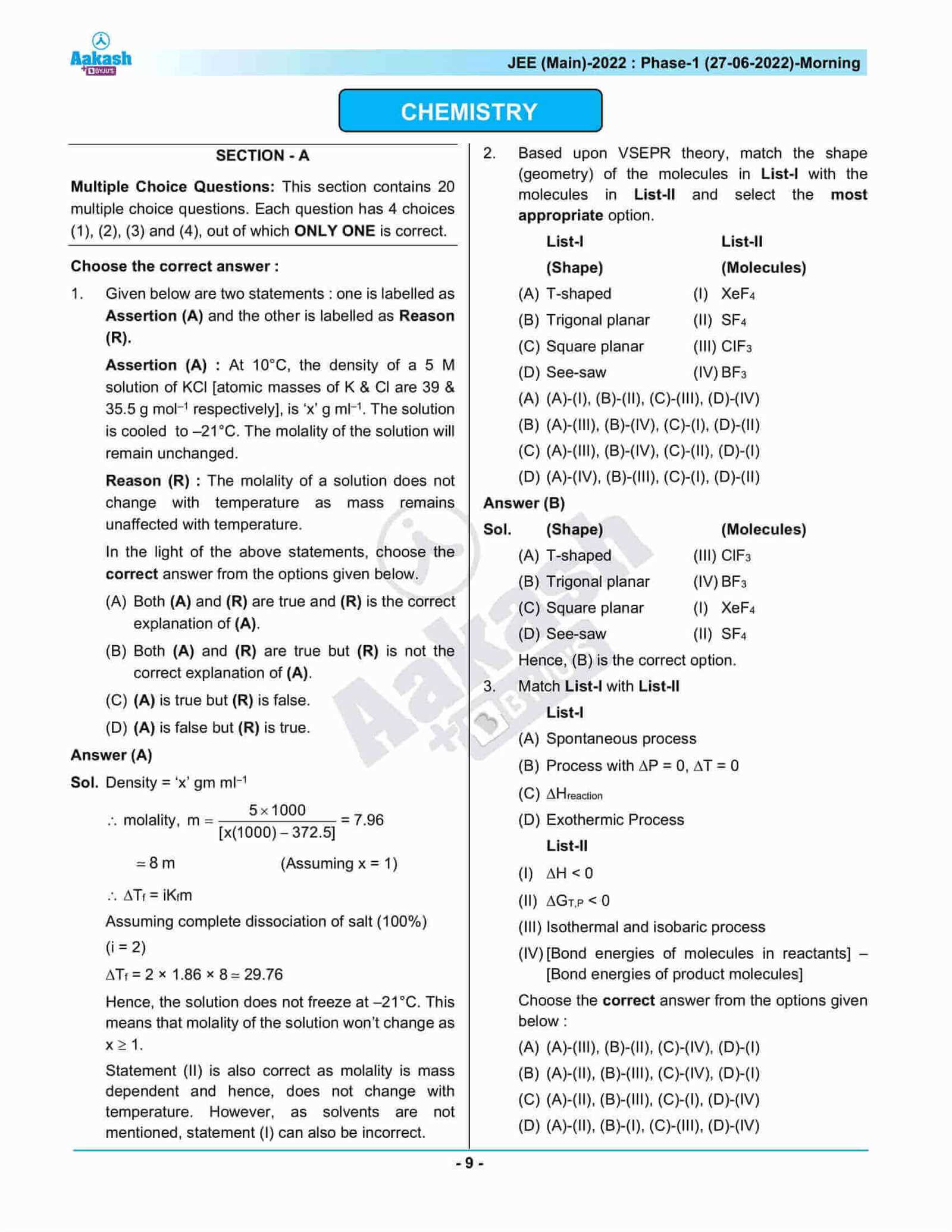
From theoretical knowledge to practical problem-solving, mastering these areas requires focus and structured revision. With a clear understanding of the material and proper techniques for handling different question types, you’ll be ready to demonstrate your full potential.
Success doesn’t just depend on memorization, but also on your ability to analyze, interpret, and reason through complex ideas. Prepare yourself with tips, resources, and insights that can turn your efforts into impressive results.
Chemistry Semester 1 Exam Answers
Achieving a high score in the first major assessment requires a strong understanding of the key concepts and effective problem-solving techniques. The ability to apply learned principles in various scenarios is essential. By focusing on mastering core topics and practicing different types of questions, you can ensure a successful outcome.
During this stage of your studies, you will encounter a range of tasks that assess both your theoretical knowledge and practical skills. Understanding the most common question formats and learning how to approach them will help reduce anxiety and improve your performance. This section provides essential tips and resources to guide you through the preparation process.
Whether it’s recalling key formulas, interpreting data, or explaining processes, practicing with diverse material will help solidify your understanding. Review the concepts you find challenging and work through problems step-by-step to enhance your problem-solving abilities. With focused revision and dedication, you can confidently navigate through the assessment and achieve the results you aim for.
Essential Topics for Semester 1 Chemistry
To excel in the initial stages of your academic journey, it’s important to focus on mastering the fundamental concepts that will be tested. Understanding these core areas will provide a strong foundation for the challenges ahead. This section covers the key subjects you should prioritize to ensure a comprehensive grasp of the material.
Key Areas to Focus On
Familiarizing yourself with the most important topics will help you approach your assignments and questions with confidence. The following table outlines the core subjects typically covered in the early part of your studies:
| Topic | Description |
|---|---|
| Atomic Structure | Understanding atoms, subatomic particles, and how they form different elements. |
| Chemical Bonding | Learning about ionic, covalent, and metallic bonds, and their properties. |
| Stoichiometry | Calculating quantities of reactants and products in chemical reactions. |
| Acids and Bases | Exploring the properties, reactions, and pH calculations of acids and bases. |
| Thermodynamics | Studying energy changes, heat, and work in chemical processes. |
Why These Topics Matter

These essential areas form the backbone of many advanced concepts you will encounter later. A solid understanding here will not only help you perform well in the first assessment but also set the stage for future learning. Dedicate time to review these topics regularly to strengthen your foundation.
How to Approach Chemistry Exam Questions
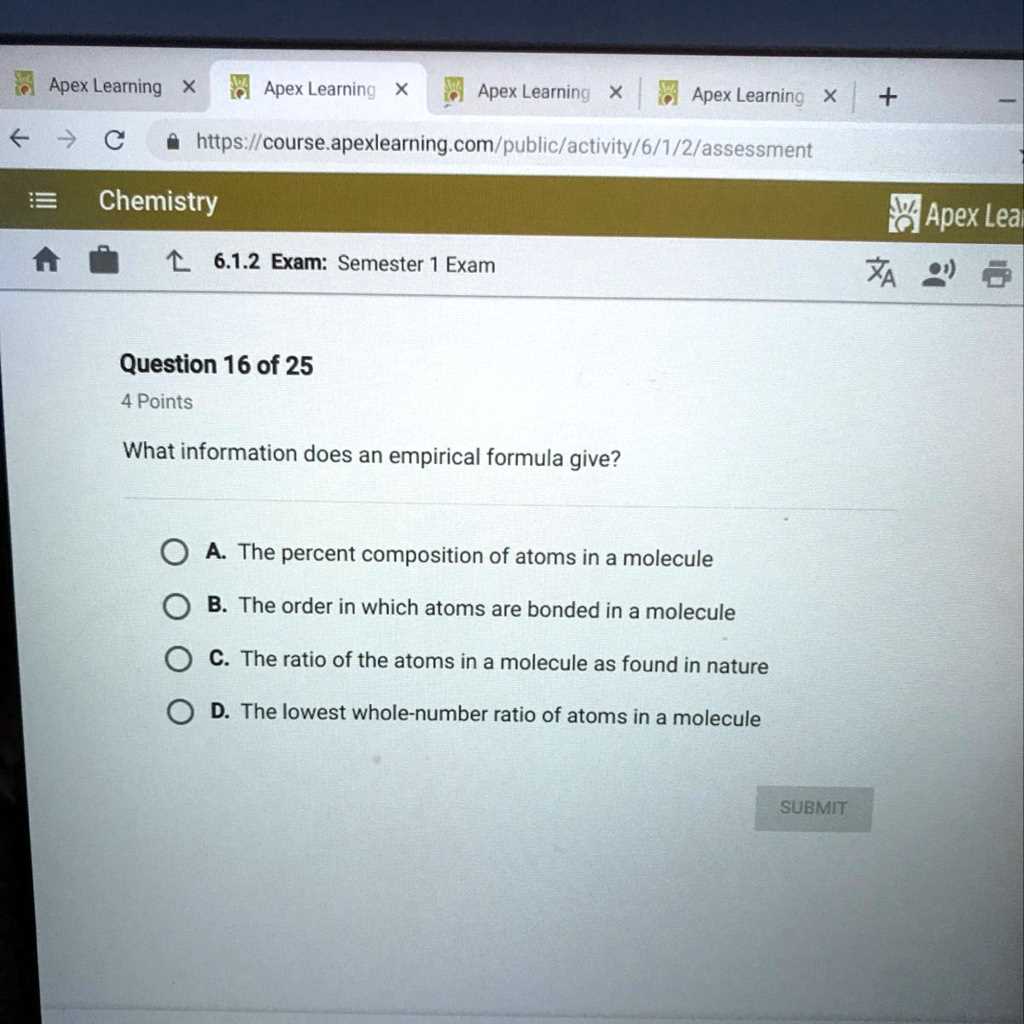
When facing a test, having a strategic approach to answering questions is just as important as understanding the material itself. Knowing how to break down each question and efficiently organize your thoughts will ensure that you address everything that’s being asked. A clear method can help manage your time and improve your accuracy, leading to better results.
Step-by-Step Approach

Read each question carefully before jumping to conclusions. Often, students miss out on key details by rushing through the instructions. Take a moment to analyze what’s being asked and highlight keywords that provide clues about the required answer.
Start with the easier questions to build confidence and ensure you secure those points early. After that, move on to more complex problems, tackling them with the same level of focus. If you encounter a challenging question, it’s okay to skip it temporarily and return to it later with a fresh perspective.
Time Management and Review
Effective time management can make a significant difference. Allocate a set amount of time to each question, ensuring you don’t spend too long on any single one. Once you’ve completed all the questions, use any remaining time to review your work. Double-check calculations and rephrase answers if necessary to ensure clarity.
Key Formulas for Chemistry Success
Mastering the fundamental equations is crucial for achieving success in assessments. These mathematical relationships allow you to solve complex problems quickly and accurately. By focusing on understanding and memorizing the key formulas, you can apply them effectively in various scenarios.
Essential Equations to Remember
Here are some of the most important formulas that will help you tackle a wide range of questions:
| Formula | Description |
|---|---|
| PV = nRT | Ideal gas law: Relates pressure, volume, temperature, and the number of moles of gas. |
| m = M × n | Mass of substance (m) is the product of molar mass (M) and the number of moles (n). |
| n = C × V | Number of moles (n) equals concentration (C) multiplied by volume (V). |
| q = mcΔT | Heat energy (q) is calculated by multiplying mass (m), specific heat capacity (c), and temperature change (ΔT). |
| E = mc² | Energy-mass equivalence formula, fundamental to understanding energy in different forms. |
How to Use Formulas Effectively
Understanding the meaning behind these formulas is just as important as memorizing them. Take time to practice applying them to different types of problems. Recognize when each equation is most appropriate, and always check your work for accuracy. By regularly using these formulas, you’ll develop a deeper understanding and improve your problem-solving speed.
Common Mistakes in Chemistry Exams
During assessments, even small mistakes can lead to significant loss of points. It’s essential to recognize common pitfalls and develop strategies to avoid them. By understanding where students often go wrong, you can better prepare yourself and approach questions with more precision.
Key Errors to Watch For
One of the most frequent mistakes is failing to read questions thoroughly. Skimming through instructions can cause you to overlook important details that change the direction of your answer. Always ensure that you fully understand what’s being asked before attempting to solve the problem.
Another common error is neglecting unit conversions. Many problems involve quantities that need to be adjusted to the correct units before performing calculations. Failing to do this can lead to incorrect results, even if the underlying concept is understood.
How to Avoid These Mistakes
To minimize these errors, take the time to read each question slowly and underline key terms. Double-check any unit conversions and always ensure that your final answer includes proper units. Practice with sample problems to develop a habit of paying attention to detail, and consider revisiting any concepts that you find challenging.
Understanding Chemical Reactions for the Test
One of the most crucial areas to master for your assessment is understanding how different substances interact and transform during reactions. A solid grasp of these processes not only helps in answering questions but also in predicting the outcomes of various chemical changes. Focusing on reaction types, balancing equations, and recognizing the factors that influence reactions will be key to your success.
Types of Reactions to Focus On
There are several types of reactions that frequently appear in assessments. Knowing how to recognize and understand each type will help you answer questions more effectively. The following table highlights the most common reaction types:
| Reaction Type | Description |
|---|---|
| Synthesis | Two or more reactants combine to form a single product. |
| Decomposition | A single compound breaks down into two or more simpler substances. |
| Single Replacement | One element replaces another in a compound. |
| Double Replacement | Two compounds react to form two new compounds, often with the formation of a precipitate. |
| Combustion | Reaction with oxygen that produces energy, typically resulting in carbon dioxide and water. |
How to Apply This Knowledge
To use this knowledge effectively, practice balancing equations and identifying the type of reaction given a set of reactants. Understanding the reactants and products in different reactions will help you predict the outcomes accurately. Additionally, familiarize yourself with the conditions that affect reactions, such as temperature, pressure, and concentration, as these often play a role in the behavior of substances during transformations.
Study Strategies for Semester 1 Chemistry
Effective preparation involves more than just reviewing notes. To truly grasp the material and perform well in assessments, you need to adopt efficient study habits that enhance both understanding and retention. Developing a clear study plan, utilizing a variety of resources, and consistently practicing are key to mastering the concepts that will be tested.
Creating a Study Plan
A well-structured study plan is the foundation for efficient learning. Breaking down the material into manageable sections allows you to focus on one topic at a time and prevents feeling overwhelmed. Here’s how to organize your study sessions:
- Prioritize topics based on difficulty and importance.
- Set specific goals for each study session (e.g., mastering a particular concept or completing practice problems).
- Review regularly to reinforce your understanding and improve retention.
Utilizing Different Resources
Maximize your understanding by using a variety of study materials. Relying on just one source may not give you a full picture of the concepts. Explore these resources:
- Textbooks and lecture notes: These provide the foundation for your studies and are your primary resources.
- Online videos and tutorials: Visual explanations can help clarify difficult concepts and offer alternative methods of understanding.
- Practice problems and quizzes: These help reinforce what you’ve learned and identify areas that need improvement.
Active Learning Techniques
Active learning is key to long-term retention. Instead of passively reading, engage with the material by:
- Summarizing key points in your own words.
- Teaching concepts to others to reinforce your understanding.
- Creating flashcards for important terms and formulas.
- Solving problems without looking at the solution to test your knowledge.
By combining a strategic study plan, diverse resources, and active learning techniques, you’ll be well-equipped to handle the challenges ahead and succeed in your assessments.
Top Resources for Chemistry Exam Preparation
To achieve the best results in your upcoming assessments, it’s essential to utilize the right tools and materials. With a wide range of resources available, selecting the ones that align with your learning style can make a significant difference in your preparation. Whether you prefer textbooks, online platforms, or interactive methods, having access to the right materials can provide a deeper understanding of key concepts and improve your performance.
Recommended Books and Guides
Books and study guides remain one of the most reliable sources for thorough exam preparation. They provide detailed explanations, practice problems, and examples that are essential for mastering complex topics. Consider using:
- Textbooks: Your primary source of information, often aligned with the curriculum and providing in-depth coverage.
- Review books: These are focused on summarizing key concepts, offering quick reference and practice questions.
- Study guides: Specially designed guides that break down material into manageable chunks and provide useful tips for test-taking strategies.
Online Resources and Platforms

Online tools offer flexibility and a wealth of interactive learning opportunities. Using websites and apps can enhance your understanding through videos, quizzes, and discussion forums. Here are some of the most useful platforms:
- Khan Academy: A free resource offering video lessons and exercises on a wide range of topics.
- Quizlet: Create custom flashcards or use pre-made sets to help with memorization of key terms and concepts.
- Coursera: Offers online courses from universities, often providing a more in-depth exploration of specific subjects.
- Wolfram Alpha: A computational knowledge engine that can help with solving problems and understanding equations.
Practice Tests and Problem Sets
Working through practice tests and problem sets is crucial for applying what you’ve learned and developing your problem-solving skills. These exercises mimic the types of questions you may encounter in assessments. Consider using:
- Past papers: Previous assessments provide insight into question formats and the types of content typically covered.
- Online quiz platforms: Websites like Quizlet and other specialized platforms offer quizzes that test your knowledge on various topics.
- Interactive problem solvers: Apps and websites that allow you to work through problems and instantly check your solutions.
By incorporating these resources into your study routine, you can enhance your understanding, refine your skills, and build confidence as you prepare for your assessments.
How to Manage Exam Time Effectively
Managing your time during a test is essential for maximizing performance and ensuring that you can complete all sections within the allotted time. The key to effective time management lies in planning ahead, staying focused, and adjusting as needed throughout the assessment. By following a structured approach, you can prevent last-minute panic and ensure that each question receives the attention it deserves.
Pre-Exam Strategies
Proper preparation before the test can set you up for success. Here are a few strategies to help you manage your time efficiently during the assessment:
- Familiarize yourself with the format: Understand the structure of the test, the types of questions, and how much time is allotted for each section.
- Practice with timed quizzes: Use practice tests to simulate the test environment and get used to working within time constraints.
- Plan your approach: Decide in advance how you will tackle each section, whether you will start with the easiest questions or tackle the hardest ones first.
During the Test

Once the test begins, it’s important to manage your time effectively in real-time. Use these techniques to stay on track:
- Allocate time per section: Read through the test and divide the time you have by the number of sections or questions. Be mindful of each section’s difficulty and adjust as needed.
- Prioritize questions: Start with questions you know well to build confidence, and then move on to more challenging ones. Skip difficult questions and return to them later if necessary.
- Keep track of time: Use a watch or the clock in the room to monitor your progress. If you’re spending too much time on one section, move on to ensure you have time for everything.
By incorporating these strategies, you can stay organized and maintain a steady pace, which will help reduce stress and improve your overall performance.
Important Equations You Must Know
Mastering key formulas is essential for solving problems effectively and quickly during any test. These equations are the building blocks of various concepts and are frequently applied to solve a wide range of questions. Understanding not only how to use them but also their derivation and application will help you approach problems with confidence.
Fundamental Equations
There are several core equations that you should memorize and be able to apply in different contexts. These form the foundation of problem-solving in this field:
- Density Equation: Density = Mass / Volume
- Ideal Gas Law: PV = nRT (where P = pressure, V = volume, n = number of moles, R = ideal gas constant, T = temperature)
- Law of Conservation of Mass: Mass reactants = Mass products
- Balancing Chemical Reactions: Ensure that the number of atoms of each element is the same on both sides of the equation.
Advanced Equations

In addition to the basic formulas, there are more advanced equations that help you solve complex problems involving reactions, energy, and rates:
- Rate of Reaction: Rate = k[A]^m[B]^n (where k is the rate constant, and m and n are the orders of the reaction with respect to the reactants A and B)
- Arrhenius Equation: k = A * e^(-Ea/RT) (where k is the rate constant, A is the pre-exponential factor, Ea is the activation energy, R is the gas constant, and T is the temperature)
- Hess’s Law: The total enthalpy change of a reaction is the sum of the enthalpy changes of the steps into which the reaction can be divided.
Familiarity with these essential equations and how they relate to one another will greatly improve your ability to solve problems under time pressure and ensure a deeper understanding of the material.
Tips for Memorizing Key Concepts
Effective memorization is essential for mastering the material and retaining information for long-term success. By applying proven strategies, you can enhance your ability to recall complex ideas and formulas. A strong understanding of key concepts requires consistent practice and active learning, which will make the material easier to grasp when it matters most.
Active Learning Techniques
Rather than passively reading through notes, engaging with the material actively can significantly improve retention. Here are some methods to help reinforce the information:
- Teach what you learn: Explaining concepts to someone else helps solidify your own understanding. If you can teach a topic clearly, you truly know it.
- Practice regularly: Repetition is key to retention. Solve problems, write out formulas, and review notes frequently to reinforce your knowledge.
- Use mnemonics: Creating memorable phrases or acronyms for complex ideas or processes can make recalling information easier. For example, using “Please Excuse My Dear Aunt Sally” to remember the order of operations.
Effective Note-Taking Strategies
Your notes are an invaluable tool for both studying and long-term recall. Here are ways to optimize your note-taking for better memorization:
- Use visual aids: Diagrams, charts, and tables can help organize information and make abstract concepts more tangible.
- Highlight key points: Emphasize essential terms, equations, and processes in your notes to make them easier to find during review.
- Break information into smaller chunks: Instead of cramming large amounts of information at once, break it down into manageable pieces to avoid feeling overwhelmed.
By adopting these strategies, you’ll be able to better retain the material, making it easier to recall and apply when needed.
Breaking Down the Periodic Table
Understanding the structure and organization of the periodic table is essential for mastering fundamental concepts in this field. It serves as a comprehensive guide to the elements, grouping them based on shared characteristics and properties. Familiarity with the table not only aids in identifying elements but also provides insight into their behavior, reactivity, and relationships with other substances.
Element Categories

The elements in the periodic table are divided into several categories based on their properties. Here are the main classifications:
- Metals: Typically good conductors of heat and electricity, they are shiny, malleable, and ductile. Most of the elements on the table are metals.
- Non-metals: These elements are poor conductors of heat and electricity. They can be found on the right side of the table and often have distinct properties, such as being brittle when solid.
- Metalloids: Located between metals and non-metals, these elements have properties that are intermediate, making them useful in semiconductors.
- Noble Gases: These gases are highly unreactive and are found in the far right column. Their full outer electron shell makes them stable.
Periodic Trends
As you explore the table, it’s important to recognize certain patterns or trends that can help predict an element’s behavior:
- Atomic Size: Generally, atomic size decreases from left to right across a period and increases down a group.
- Electronegativity: This trend indicates the tendency of an atom to attract electrons when bonded to another atom. It increases across a period and decreases down a group.
- Ionization Energy: The energy required to remove an electron from an atom. Ionization energy generally increases across a period and decreases down a group.
By understanding these categories and trends, you’ll be better equipped to predict how elements will behave in reactions and their interactions with one another.
How to Tackle Multiple Choice Questions
Multiple choice questions are a common assessment format designed to test your understanding of key concepts. While they may seem straightforward, the ability to approach them strategically can greatly improve your performance. By applying certain techniques, you can increase your chances of selecting the correct answer and avoid common pitfalls.
Reading the Question Carefully
The first step in answering multiple choice questions effectively is to carefully read the entire question. Here’s what you should do:
- Understand the question: Focus on the specific information being asked. Don’t get distracted by unnecessary details.
- Look for keywords: Words like “always,” “never,” “best,” and “most” can change the meaning of the question, so be mindful of them.
- Identify the main concept: Recognize what the question is testing, whether it’s a definition, concept, or application of knowledge.
Eliminating Incorrect Options
One effective strategy is to eliminate the obviously incorrect options before making a final selection:
- Cross out obvious errors: If an option is clearly false or irrelevant, discard it immediately.
- Consider the remaining choices: Analyze each of the remaining answers. Compare them against your understanding of the topic to see which one fits best.
- Look for patterns: Sometimes, if two choices seem similar, one of them might be the right answer. Pay attention to slight differences.
By applying these strategies, you can tackle multiple choice questions with confidence and make better, more informed decisions.
What to Do on Exam Day
The day of an assessment is crucial, as it sets the stage for how you’ll perform under pressure. It’s not just about what you do during the test itself but how you prepare beforehand. A calm, focused approach can make all the difference, ensuring that you are fully prepared to tackle the challenges that await you.
On the day of your assessment, it’s important to start early, both physically and mentally. Begin by ensuring you have all the necessary materials, such as pens, pencils, identification, and any allowed resources. Make sure you’re familiar with the location and any specific instructions provided ahead of time.
As you get closer to the start time, take a moment to relax and clear your mind. Avoid last-minute cramming, as this can cause unnecessary stress and diminish your focus. Instead, do something light and calming–maybe a short walk or a quick review of key concepts that are fresh in your memory.
When you enter the assessment space, focus on staying calm. Breathe deeply and take a moment to settle in. Read all instructions thoroughly, and don’t hesitate to ask if something is unclear. Once you begin, manage your time wisely and approach each question with the strategies you’ve practiced.
Reviewing Lab Work for the Exam
Hands-on experiments and practical tasks often play a key role in the assessment process. It’s essential to review and fully understand the work you’ve completed in the lab, as it forms a significant part of your preparation. By reflecting on past experiments, you can reinforce your knowledge of key concepts and methodologies, and avoid common pitfalls during the test.
To effectively review your lab work, consider the following steps:
- Review Lab Reports: Go over any reports or notes you’ve written. Ensure that you understand every step of the process, from the initial hypothesis to the final results. Focus on the reasoning behind each experiment and how the outcome relates to theoretical knowledge.
- Understand Procedures: Familiarize yourself with the methods and techniques you used. Understand why each procedure was chosen and what results were expected. This helps in recalling what to do in a similar situation.
- Identify Key Observations: Focus on the key observations and measurements recorded during your experiments. Be sure you understand how they contribute to the conclusion and what any discrepancies might indicate.
- Practice with Sample Problems: If available, practice solving questions related to the lab work. These might include analyzing results, calculating yields, or explaining the outcomes of specific reactions.
By following these strategies, you can ensure that your lab work review is thorough and beneficial, enabling you to tackle practical-related questions confidently in your assessment.
Best Revision Methods for Chemistry Exams
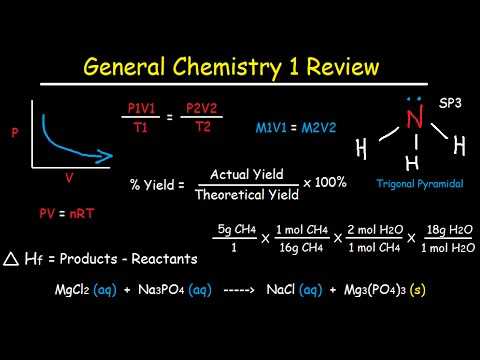
Effective revision requires more than just reading through notes. It involves a variety of strategies that help reinforce knowledge, identify gaps, and build confidence. By using a range of revision techniques, you can enhance your understanding and retention of key concepts, making the revision process more productive and focused.
Here are some of the best revision methods for mastering the material:
| Revision Method | Description | Benefits |
|---|---|---|
| Active Recall | Test yourself on key concepts without looking at your notes. Write down everything you can remember, and then check for accuracy. | Improves long-term retention and helps identify weak areas. |
| Spaced Repetition | Review material over increasing intervals of time, ensuring that you come back to concepts before forgetting them. | Strengthens memory by reinforcing knowledge at optimal intervals. |
| Mind Mapping | Create visual diagrams that show the connections between different topics. This helps to organize your thoughts. | Helps you see the bigger picture and makes complex information easier to understand. |
| Practice Problems | Work through past papers or practice questions regularly. Focus on questions that challenge your knowledge. | Builds confidence and improves problem-solving skills under time pressure. |
| Group Study | Study with a group of peers. Discuss complex concepts and quiz each other on material. | Provides different perspectives and helps reinforce understanding through teaching others. |
By incorporating these methods into your revision routine, you can maximize your preparation and feel well-prepared for your upcoming assessments.