
The process of analyzing specific reactions when certain substances are exposed to heat is a fundamental aspect of chemistry. This approach provides valuable insight into the nature of different chemical elements based on the unique hues they emit under certain conditions. Understanding how different elements respond to high temperatures is essential for accurately identifying them in various settings, from academic experiments to industrial applications.
By observing the specific colors that appear when elements interact with intense heat, one can deduce important characteristics of the substance involved. These reactions are not only visually striking but also serve as a reliable method for distinguishing between different chemical components. The varying shades emitted during these reactions can reveal a great deal about the composition of a substance, making this process an essential tool in the field of chemistry.
In this section, we will explore the key techniques, procedures, and underlying principles behind these color reactions, highlighting their significance in the scientific world.
Flame Test for Metals Lab Answers
When certain substances are subjected to high temperatures, they produce characteristic colors that can be used to identify their chemical composition. This phenomenon is a powerful tool in scientific analysis, offering a visual representation of an element’s properties. By carefully observing the hues emitted during these reactions, one can determine which elements are present in a sample, making it an essential technique in both educational and industrial chemistry.
Each element, when heated, emits a distinct color due to the excitation of electrons within its atoms. These colors are the result of energy released as the electrons return to their lower energy states. This reaction varies between different substances, providing a clear, identifiable marker for each element involved. The use of this technique is common in various fields, from simple educational exercises to more complex analyses in scientific research.
In this section, we will explore how to interpret the visual cues produced during these reactions. The identification process is straightforward once you are familiar with the colors associated with specific elements. Understanding these patterns is key to accurately determining the components of an unknown sample.
How Flame Tests Work in Metal Identification
The process of identifying substances based on the colors they emit when exposed to heat relies on the excitation of electrons within their atoms. When a sample is heated, the energy provided causes the electrons to move to higher energy levels. As they return to their original state, they release energy in the form of light, which manifests as distinct colors. These colors act as a fingerprint for the elements present, making it possible to identify them based on their unique light emissions.
Principles Behind Color Emission
The colors produced during heating are a direct result of the unique arrangement of electrons in the atoms of different elements. Each element has a specific energy level configuration, which determines the wavelength of light it emits when its electrons fall back to lower levels. This is why elements like sodium produce a bright yellow light, while copper can emit green or blue shades. Understanding these patterns allows chemists to distinguish between various substances based on the color of the light they emit.
Application in Identifying Elements
This method is commonly used in both educational and professional environments for element identification. By observing the color produced when a sample is heated, one can determine the presence of specific elements, such as potassium, lithium, or calcium. The simplicity and reliability of this technique make it an invaluable tool in various chemical analyses and experiments.
Key Reactions and Colors Observed
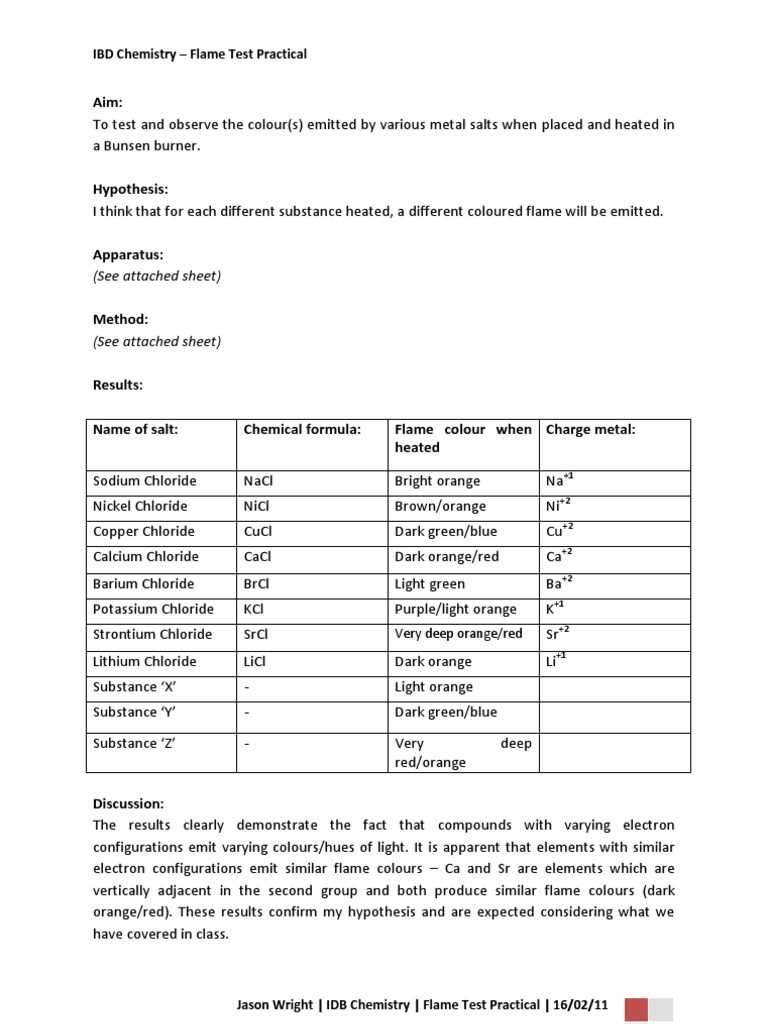
When elements are heated, they undergo specific reactions that result in distinct colors being emitted. These color changes are due to the release of energy as electrons in the element’s atoms transition between energy levels. The emitted light provides a unique signature that allows chemists to identify the substance. Each element produces a characteristic color, which can be observed and used for identification in a variety of experiments and practical applications.
Common Color Emissions
The following colors are commonly observed when various substances are heated:
- Sodium: Bright yellow
- Potassium: Light lilac or pale violet
- Calcium: Orange-red
- Strontium: Red
- Cupric compounds: Green
- Lithium: Crimson red
- Barium: Pale green
Factors Influencing Reactions
The intensity and exact shade of the colors produced can be influenced by several factors, such as:
- Temperature: Higher temperatures often lead to more vivid color emissions.
- Purity of the sample: Impurities in the substance can alter the color of the light emitted.
- Concentration: The concentration of the element in the sample can affect the intensity of the color.
Understanding these factors is important for accurate identification and consistent results when performing these reactions.
Common Metals Tested in Flame Reactions
Various substances are commonly analyzed by heating them to observe the specific colors they emit. These substances, often metallic elements, react in distinct ways when subjected to heat, releasing characteristic colors that help in their identification. By understanding the color patterns associated with each element, scientists and students alike can quickly determine which elements are present in a sample.
List of Commonly Tested Elements
The table below shows some of the most common elements analyzed using heat reactions, along with the typical color they produce during the process:
| Element | Color Emitted |
|---|---|
| Sodium | Bright yellow |
| Potassium | Light violet |
| Calcium | Orange-red |
| Strontium | Crimson red |
| Lithium | Deep red |
| Barium | Pale green |
| Copper | Blue-green |
Significance of Common Elements
These elements are commonly chosen for their clear and distinct color emissions, making them ideal for this type of analysis. The ability to identify them based on color allows for quicker, more efficient identification in various practical settings, including educational demonstrations, chemical analysis, and industrial processes. Understanding these reactions is an essential part of elemental analysis in chemistry.
Scientific Principles Behind Flame Tests
When certain substances are exposed to high temperatures, they undergo a transformation that results in the emission of visible light. This phenomenon is a key part of understanding the behavior of atoms and molecules under heat. The light emitted during this process is not random but instead follows distinct patterns depending on the substance being heated. These patterns are determined by the energy levels of the electrons in the atoms of the element being analyzed.
As heat is applied to a substance, the energy excites the electrons, causing them to move from a lower energy state to a higher one. When the electrons return to their original state, they release the excess energy in the form of light. The wavelength, or color, of the emitted light is specific to the element because each element has a unique arrangement of electrons and energy levels. This is why each element produces a characteristic color when heated.
The study of these color emissions, known as atomic emission spectra, forms the scientific basis behind this analytical technique. The colors emitted are used to identify elements because they act as a “fingerprint” for each element. By analyzing these wavelengths, scientists can determine the composition of a substance based on the light it emits under heat.
Materials Needed for a Flame Test
To conduct a reaction that reveals the unique light emitted by various substances when heated, certain materials are required. The setup needs to ensure that the elements are properly heated and that their emitted colors can be clearly observed. Some of these materials are used for safely applying heat, while others help hold the substances in place during the experiment. Proper preparation and the correct materials are crucial for obtaining accurate and reliable results.
Essential Equipment
The following items are commonly needed to carry out this type of analysis:
- Heat Source: A Bunsen burner or similar device for applying controlled heat.
- Wire Loop: A metal loop, often made of platinum or nichrome, used to hold the sample.
- Sample Substances: A selection of compounds or elements to analyze (e.g., salts of sodium, potassium, calcium).
- Chemical Solutions: Sometimes, chemicals like hydrochloric acid are used to dissolve samples before heating.
- Safety Equipment: Protective gear, including safety goggles and lab coats, to ensure safety during the experiment.
Additional Tools for Precision
To enhance the accuracy of observations, the following tools may also be helpful:
- Heat-resistant Surface: A stable surface that can withstand the high temperatures used during the reaction.
- Color Chart: A chart to compare and identify the colors emitted by different substances.
- Gas Supply: In some setups, a regulated gas supply may be needed to control the flame temperature.
Using these materials properly ensures the process is both safe and effective, allowing for clear identification of the chemical elements based on their unique color emissions when heated.
Step-by-Step Procedure for Conducting the Test
To identify the chemical elements in a sample based on the colors they emit when heated, it’s important to follow a precise sequence of steps. This ensures that the reactions are consistent and the colors produced are observed clearly. The process involves preparing the necessary materials, carefully heating the sample, and observing the emitted light. By following these steps, you can accurately determine the composition of the sample being analyzed.
Preparation and Setup
Before beginning the analysis, make sure to gather all the necessary materials and set up the work area properly:
- Ensure the work area is clean and free of flammable materials.
- Wear appropriate safety equipment, including safety goggles and a lab coat.
- Set up the heat source, such as a Bunsen burner, on a stable, heat-resistant surface.
- Prepare the sample by attaching it to the wire loop or dissolving it in a suitable solution, depending on the type of element being analyzed.
Conducting the Analysis
Once the setup is complete, follow these steps to carry out the procedure:
- Dip the wire loop into the sample to collect a small amount of the substance.
- Place the wire loop into the center of the flame, holding it steady and allowing it to heat evenly.
- Observe the color emitted by the sample as it heats. Take note of the color immediately, as it may change or fade over time.
- Repeat the procedure with different samples, cleaning the wire loop thoroughly between each analysis to avoid contamination.
- Record the color observed for each substance and compare it to known color emissions to identify the elements present.
By following this procedure carefully, you can ensure that the results are reliable and that the chemical components of the sample are correctly identified based on their characteristic color emissions when exposed to heat.
Interpreting Flame Test Results Accurately
Accurately interpreting the results of this experiment is crucial for identifying the elements in a sample. The colors emitted during the process provide valuable clues, but several factors must be considered to ensure correct identification. These factors include the intensity and stability of the color, the presence of other elements that might interfere, and the comparison to known color patterns for various elements.
The first step in accurate interpretation is to observe the color emitted by the sample as it heats. Some elements produce intense, vibrant colors, while others emit more subtle hues. It is important to note any changes in the color over time, as certain elements might show a shift in shade as they burn. For instance, some substances may initially display one color, but as they continue to react, the color might fade or transform, providing additional information about the element’s behavior.
Key Considerations for Accurate Interpretation:
- Color Intensity: The intensity of the emitted color can help indicate the concentration of the substance in the sample. More concentrated elements often produce brighter, more vivid colors.
- Presence of Interfering Elements: Be aware of the potential for other substances in the sample to influence the observed color. For example, contaminants or impurities might alter the expected color.
- Comparison with Known Color Standards: Compare the observed color with established standards or a reference chart to accurately identify the element. Each element emits a specific color pattern that can be matched to known emission spectra.
- Repeatability: Ensure that the test is performed multiple times with consistent results. If the same color is observed in multiple trials, this strengthens the reliability of the interpretation.
By carefully considering these factors and comparing the results to known color patterns, accurate identification of the elements can be achieved. This method allows scientists to perform elemental analysis based on the unique characteristics of each substance’s emission under heat.
Identifying Alkali and Alkaline Earth Metals
Alkali and alkaline earth substances can be distinguished by the distinct colors they emit when subjected to heat. These groups of elements, found in the first two columns of the periodic table, each produce characteristic hues that can be used to identify them in a sample. While alkali elements tend to display vibrant and unique colors, alkaline earth substances often produce more subdued, yet equally identifiable, shades when exposed to high temperatures.
Alkali substances, which include elements like lithium, sodium, and potassium, are known for their vivid colors. When exposed to heat, they often emit intense hues ranging from red to violet, each one associated with a specific element. These elements have a single electron in their outer shell, which contributes to the ease with which they are excited, producing bright emissions.
On the other hand, alkaline earth elements, such as calcium, strontium, and barium, produce a different range of colors, often more subtle but still distinct. Calcium can produce an orange-red glow, strontium emits a red color, and barium typically gives off a green hue. These elements, with two electrons in their outer shell, behave somewhat differently under heat but still provide clear visual identifiers that aid in their recognition.
Understanding the unique color patterns of these groups not only aids in their identification but also provides valuable insights into their chemical properties and behavior when heated. By carefully observing the emitted light, it is possible to distinguish between alkali and alkaline earth elements based on the color they release during the heating process.
Flame Test for Copper and its Compounds
Copper and its compounds exhibit distinctive behaviors when heated, allowing for their identification based on the colors they emit during heating. The color of the emitted light can reveal important information about the presence of copper in a sample, making it possible to differentiate copper compounds from other substances. By carefully observing the specific hue produced, it is possible to identify copper and some of its commonly encountered compounds.
Copper’s Color in Heat
When copper salts are exposed to heat, they emit a characteristic green or blue flame, depending on the specific compound. Copper(II) compounds, such as copper sulfate, often produce a bright green color, while copper(I) compounds may show a more bluish tint. The intensity and shade of the emitted color can vary, providing additional clues about the specific type of copper compound present.
Common Copper Compounds and Their Colors
The following table summarizes the colors produced by different copper compounds when heated:
| Compound | Color Emitted |
|---|---|
| Copper(II) sulfate | Green |
| Copper(II) chloride | Green |
| Copper(I) chloride | Blue |
| Copper(I) oxide | Red |
These color patterns are a result of the way copper ions interact with heat, and can be used to identify the presence of copper in a sample or to distinguish between different copper compounds. The specific colors produced are tied to the energy levels of the electrons in the copper atoms and ions, which absorb energy and re-emit it in the form of visible light. By comparing the observed colors to known references, the copper compound in question can be identified with a high degree of accuracy.
Flame Test for Sodium and Potassium
Sodium and potassium are both alkali elements that produce distinct and easily recognizable colors when subjected to heat. The unique hues emitted by these elements are a result of their electron configurations and are useful in distinguishing them from other substances. By observing the color produced during heating, it is possible to identify sodium and potassium in various compounds with high precision.
Sodium is known for producing a bright, intense yellow color when heated. This is one of the most well-known flame colors and can be observed with the naked eye even from a distance. The yellow hue is caused by the excitation of sodium’s outer electron, which releases energy as visible light when it returns to its ground state. This characteristic yellow flame is consistent across various sodium compounds, making it a reliable indicator of the element.
Potassium, in contrast, emits a pale lilac or light purple color when exposed to heat. This color is less intense than sodium’s yellow but still quite noticeable. The purple hue is caused by the excitation of potassium’s outermost electron, similar to sodium, but with a different energy release, resulting in a distinct color. Potassium compounds such as potassium chloride and potassium nitrate produce this unique shade during the heating process.
The ability to distinguish between sodium and potassium based on their flame colors is valuable in both qualitative analysis and laboratory experiments. While the yellow of sodium is more vivid and dominant, potassium’s purple color is softer and can sometimes be masked by more intense light sources, requiring careful observation to identify accurately.
Why Flame Colors Vary for Different Metals
The variation in flame colors produced by different elements is a result of the way their electrons respond to heat. When a substance is heated to a high temperature, its electrons absorb energy and jump to a higher energy level. As the electrons return to their original state, they release energy in the form of light. The color of this light depends on the specific energy differences between the electron levels in the atoms of each element. This explains why different elements produce unique colors when exposed to heat.
Factors Influencing Flame Colors
The specific color observed is determined by several factors related to the atom’s structure, including:
- Energy Levels: The amount of energy required to excite electrons differs between elements. This results in the emission of light at different wavelengths, which corresponds to different colors.
- Electron Transitions: The specific transitions of electrons in an atom determine the frequency and energy of the emitted light, influencing the color produced during heating.
- Atomic Size: The size of the atoms also plays a role in determining the color. Larger atoms often have lower energy differences between their electron levels, which can result in different wavelengths of light being emitted.
Common Examples of Elemental Flame Colors
Here are some examples of common elements and the colors they produce when heated:
- Sodium: Intense yellow
- Potassium: Light purple
- Calcium: Orange-red
- Barium: Green
- Copper: Green or blue, depending on the compound
These colors are unique to each element due to the specific characteristics of their atomic structure and the way their electrons interact with heat. Understanding these differences allows scientists to identify the presence of specific elements in various samples through simple visual observation of the emitted light.
Factors That Affect Flame Test Outcomes
The results of a heat-induced color emission experiment can be influenced by several variables. While the general principle remains consistent – that elements emit specific colors when heated – certain factors can impact the intensity and accuracy of the observed results. Understanding these factors is crucial for ensuring reliable and precise identification of elements.
Key Variables Impacting Results
Several conditions can alter the outcome of these observations. The most important factors include:
- Sample Purity: Contaminants or impurities present in the sample can change the color of the emitted light. The presence of other elements might mix with the desired emission spectrum, leading to inaccurate results.
- Temperature: The temperature at which the sample is heated plays a critical role. Higher temperatures can lead to more intense colors, while lower temperatures may not excite electrons sufficiently to produce noticeable color changes.
- Flame Characteristics: The type of heat source and its consistency can also influence the results. A flame with a higher or lower temperature, or one with varying intensity, may not allow for accurate observations of the characteristic color of the sample.
- Time of Exposure: The duration for which the sample is exposed to the heat source can affect the brightness and clarity of the emitted color. Insufficient time might result in faint colors that are difficult to observe.
- Environmental Factors: Factors such as surrounding lighting, the presence of other gases, and humidity can impact the visibility and consistency of the observed results.
How Each Factor Affects Color Emission
Here’s a breakdown of how each factor can influence the flame color observed:
| Factor | Effect on Result |
|---|---|
| Sample Purity | Can introduce additional colors or interfere with the characteristic color. |
| Temperature | Higher temperatures typically lead to brighter, more intense colors. |
| Flame Characteristics | Inconsistent or weaker flames may not produce the desired colors accurately. |
| Time of Exposure | Short exposure times may result in faint colors, making them hard to distinguish. |
| Environmental Factors | External lighting and conditions can distort the visibility of emitted colors. |
By controlling these variables, it is possible to obtain more reliable results, ensuring that the color emitted during heating corresponds accurately to the element being tested. It is important to consider each factor when interpreting the outcomes to avoid misidentification.
Applications of Flame Tests in Real-World Chemistry

The technique of observing color changes when elements are heated plays an important role in various fields of chemistry. While it is often considered a fundamental method for identifying substances in a controlled setting, its applications extend beyond the classroom and into industries and research. The distinct colors emitted by different substances when heated make this technique a valuable tool for several practical purposes.
Key Areas of Application
Several industries and research areas benefit from the use of color emission experiments, utilizing the distinct light patterns to identify elements and monitor chemical reactions. Some of the most common applications include:
- Analytical Chemistry: This method is used in chemical analysis to detect and identify the presence of specific elements in samples. It provides a quick, low-cost alternative to more complex instrumentation in some cases.
- Environmental Monitoring: Color emission techniques are useful in tracking pollutants and hazardous materials. They allow for real-time monitoring of elements in air, water, and soil, helping to assess environmental contamination levels.
- Quality Control in Manufacturing: Industries such as glass production and ceramics use this technique to ensure the purity of raw materials and the consistency of final products. The colors emitted help confirm that the correct substances are present during production.
- Fireworks and Pyrotechnics: This technique is a cornerstone of creating vibrant, colorful displays in fireworks. By mixing various compounds, manufacturers can control the colors produced in each explosion.
- Educational Purposes: Flame color experiments remain a staple in chemistry classrooms for demonstrating fundamental concepts of atomic structure and electron excitation, making it an important tool for teaching and engaging students.
Benefits and Limitations in Practical Use
While this method offers a fast and simple approach for certain applications, it also has its limitations. For instance, it may not be suitable for detecting trace amounts of elements or for samples that are complex mixtures. However, its low cost and ease of use make it a valuable option in many settings where quick, preliminary results are needed.
By understanding the various applications of color emission experiments, scientists and industries can make informed decisions about when and how to use this technique for effective results in their work.
Safety Tips for Performing Flame Tests
When conducting experiments that involve heating substances, safety should always be the top priority. Proper precautions must be taken to ensure a safe environment, as the process can involve high temperatures, flammable materials, and potentially hazardous chemicals. By following key safety practices, you can minimize risks and ensure the experiment is both effective and secure.
Essential Safety Precautions
Before beginning any experiment involving heating, it’s important to follow safety guidelines. Here are some critical steps to keep in mind:
- Wear Proper Protective Gear: Always wear safety goggles, gloves, and a lab coat to protect your eyes, hands, and skin from potential burns or splashes.
- Ensure Adequate Ventilation: Perform the experiment in a well-ventilated area to avoid inhaling any fumes or gases produced during the heating process.
- Check Equipment Functionality: Make sure all equipment, such as Bunsen burners or other heating devices, are in proper working condition. Inspect gas connections and burners for leaks before use.
- Avoid Flammable Materials: Keep flammable objects away from the heating area. Make sure there is nothing nearby that could catch fire in the event of an accidental spark.
- Handle Chemicals with Care: Use tongs or appropriate tools to handle any chemicals or substances that are being heated. Never touch hot equipment with bare hands.
- Have an Emergency Plan: Be familiar with the location of fire extinguishers, eyewash stations, and first aid kits. Know how to react in case of an emergency.
Best Practices During the Experiment
While performing the experiment, ensure that you follow these best practices to minimize risk:
- Work in Pairs: Always conduct experiments with a partner, especially when working with flammable substances, so assistance is available in case of accidents.
- Never Leave the Heating Area Unattended: Always stay at the experiment’s location while heating substances to prevent accidents that may occur when no one is around to respond quickly.
- Dispose of Materials Properly: After completing the experiment, dispose of chemicals and materials according to your institution’s safety guidelines to prevent contamination or reaction with other substances.
By following these essential safety tips, you can ensure a safer and more successful experience when performing experiments that involve heating substances and observing their reactions.