The movement of substances across cell membranes is a crucial biological process that affects various aspects of life. This mechanism, which is fundamental to the functioning of living organisms, ensures that cells maintain a proper balance of essential compounds. Understanding how molecules travel in and out of cells helps explain vital functions such as nutrient absorption, waste removal, and overall cell health.
At its core, this process involves the movement of water and other molecules through a selective barrier. This selective permeability allows cells to regulate what enters and exits, depending on the needs of the organism. By exploring how this natural system works, we gain insights into cellular behavior and its broader implications for both plant and animal life.
In this section, we break down the essential steps and factors involved in this important biological process. We will look at key examples, real-world applications, and the effects of this movement on different types of cells. This knowledge provides a deeper understanding of how living organisms manage internal stability and interact with their environment.
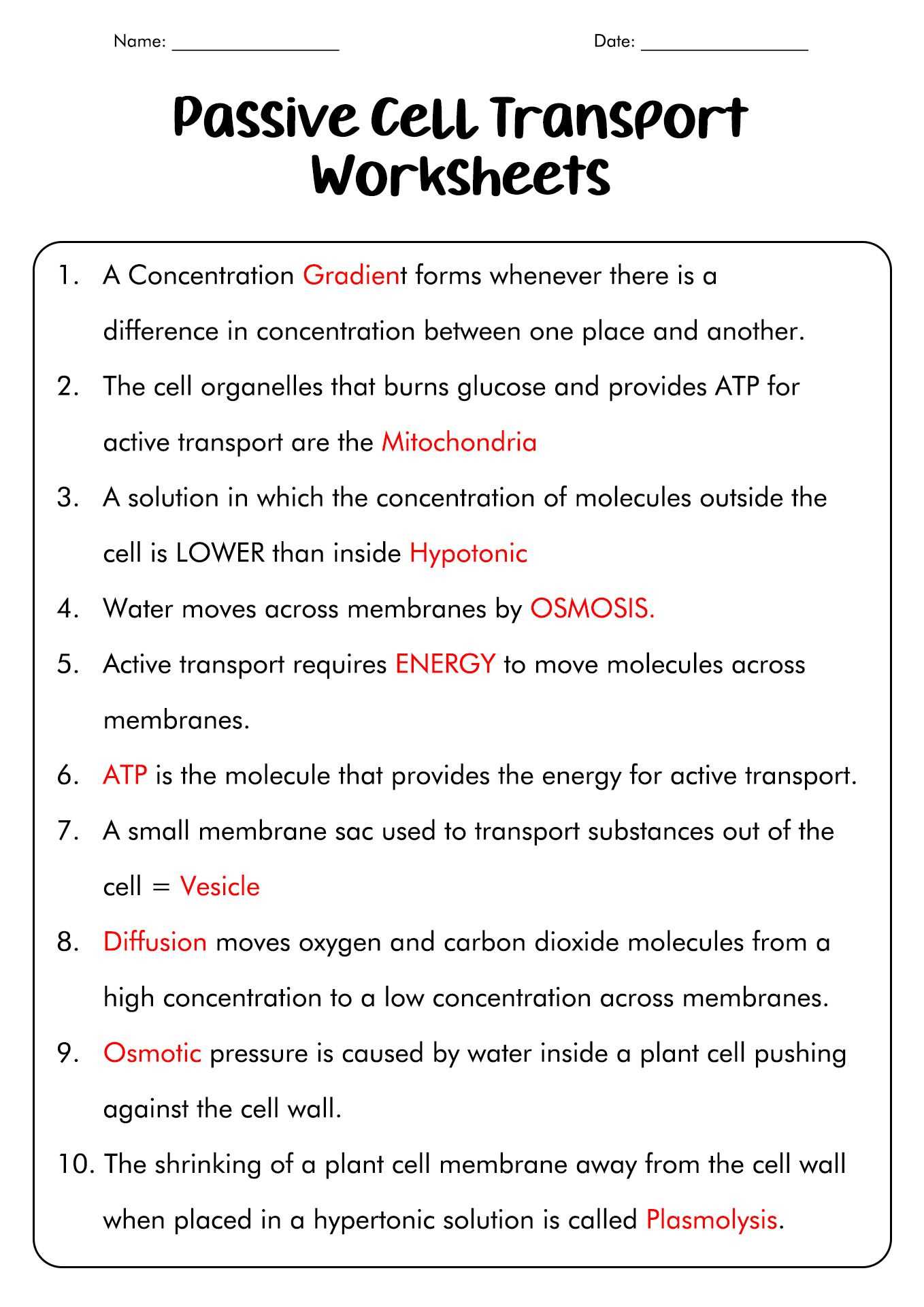
Worksheet 8 Osmosis Basic Concepts Answers
In this section, we will delve into the key principles behind the movement of water and solutes across cell membranes. This fundamental process plays a critical role in maintaining the balance of substances within cells and organisms, affecting a wide range of biological functions. By exploring the driving forces behind this movement, we can better understand how cells manage resources, react to changes in their environment, and maintain stability.
Understanding the Key Factors
The process relies on several important factors that influence how substances move in and out of cells:
- Concentration Gradient: The difference in concentration of substances on either side of the membrane.
- Membrane Permeability: How easily certain molecules can pass through the cell membrane.
- Pressure: The force exerted by the movement of molecules, which can influence the rate of transfer.
Common Examples and Applications
This process is not just theoretical–its effects can be observed in everyday life and in various scientific experiments. Some common examples include:
- Plant Watering: Understanding how water moves into plant cells helps us optimize watering techniques.
- Medical Treatments: Dialysis machines use similar principles to filter waste from the blood.
- Food Preservation: The use of salt or sugar in preservation techniques helps draw moisture out of food, preventing microbial growth.
Understanding Osmosis in Simple Terms
At the heart of many biological processes is the movement of water and other substances across cell membranes. This process helps cells regulate their internal environment, balancing essential compounds and removing waste. By understanding the driving forces behind this movement, we can better appreciate how cells manage to maintain life under various conditions.
The process can be described as a natural flow of substances from areas of higher concentration to areas of lower concentration. This flow continues until balance, or equilibrium, is achieved. The movement depends on factors like the size of molecules, the permeability of the membrane, and external conditions such as temperature and pressure.
| Factor | Effect on Movement |
|---|---|
| Concentration Difference | Substances move from areas with more molecules to areas with fewer molecules. |
| Membrane Permeability | Determines which substances can pass through the membrane and at what rate. |
| External Pressure | Can either assist or hinder the movement of substances through the membrane. |
Key Principles of Osmosis Process
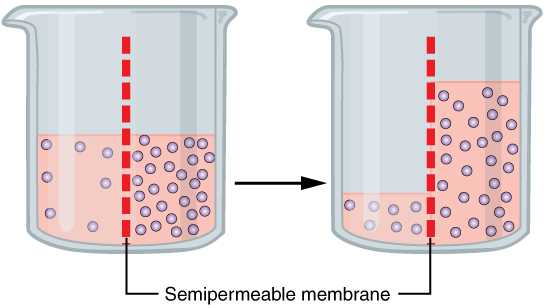
At its core, this biological mechanism involves the movement of substances across a selective barrier, ensuring that cells maintain the right balance of water, ions, and other molecules. The primary principle is that materials naturally move from areas of higher concentration to areas of lower concentration, striving for equilibrium. This process is critical for maintaining cell health, regulating volume, and enabling proper function within living organisms.
The movement is influenced by several factors, which include the type of material being transported, the properties of the membrane, and environmental conditions. These elements dictate how substances move in and out of cells, making it a highly regulated process essential to life.
| Principle | Description |
|---|---|
| Selective Permeability | The membrane allows only certain molecules to pass through, controlling the movement of substances. |
| Concentration Gradient | Substances move from areas of higher concentration to lower concentration until equilibrium is reached. |
| Equilibrium | The process continues until there is an equal distribution of substances on both sides of the membrane. |
What Happens During Osmosis
When substances move across a cell membrane, it results in a shift that ensures a balance of water, nutrients, and other molecules. This process occurs automatically, driven by differences in concentration, where molecules naturally seek to equalize themselves across the barrier. The movement doesn’t require energy and continues until an equilibrium is reached, allowing the cell to function properly.
During this process, several key actions take place:
- Water Movement: Water molecules travel from areas of high concentration to areas of low concentration to balance the concentration of solutes.
- Solute Interaction: Solutes, such as salts or sugars, move depending on the permeability of the membrane and the concentration gradient.
- Membrane Selectivity: The membrane’s properties determine which molecules can pass through and how easily they do so.
The effects of this movement are most noticeable in cells, where changes in water volume can influence their shape and functionality. For example, when water enters a plant cell, it can cause the cell to become turgid, giving the plant structure and stability. Similarly, in animal cells, the balance of water is crucial for maintaining cell size and function.
Role of Semi-Permeable Membranes
Semi-permeable membranes play a crucial role in regulating the movement of substances in and out of cells. These specialized barriers allow certain molecules to pass through while blocking others, maintaining the delicate balance needed for cell function. This selective permeability ensures that essential molecules, such as nutrients and water, enter the cell, while waste products can be removed efficiently.
The properties of these membranes make them essential in processes that control the internal environment of cells. For example, they can regulate the flow of water, ions, and other compounds, depending on their size, charge, and concentration gradient. Without this selective barrier, cells would struggle to maintain homeostasis and could be overwhelmed by unwanted substances or deprived of necessary materials.
Exploring Osmotic Pressure and its Impact
Osmotic pressure is a fundamental force that governs the movement of water across cell membranes. It arises from the difference in concentration of solutes on either side of a membrane, and it plays a significant role in maintaining fluid balance within cells and tissues. This pressure is crucial for regulating the flow of water into and out of cells, influencing their shape and volume.
The impact of osmotic pressure is seen in many biological processes, particularly in plants and animals. In plants, osmotic pressure helps maintain turgidity, giving cells the necessary structure to support the plant. In animal cells, the regulation of osmotic pressure ensures that cells neither shrink nor swell excessively, preventing damage and maintaining proper function. Any imbalance in this pressure can lead to serious consequences, such as dehydration or swelling of cells.
Understanding osmotic pressure provides valuable insight into how organisms control water movement, adjust to environmental conditions, and maintain internal stability. This knowledge is essential in various fields, including medicine, agriculture, and biotechnology.
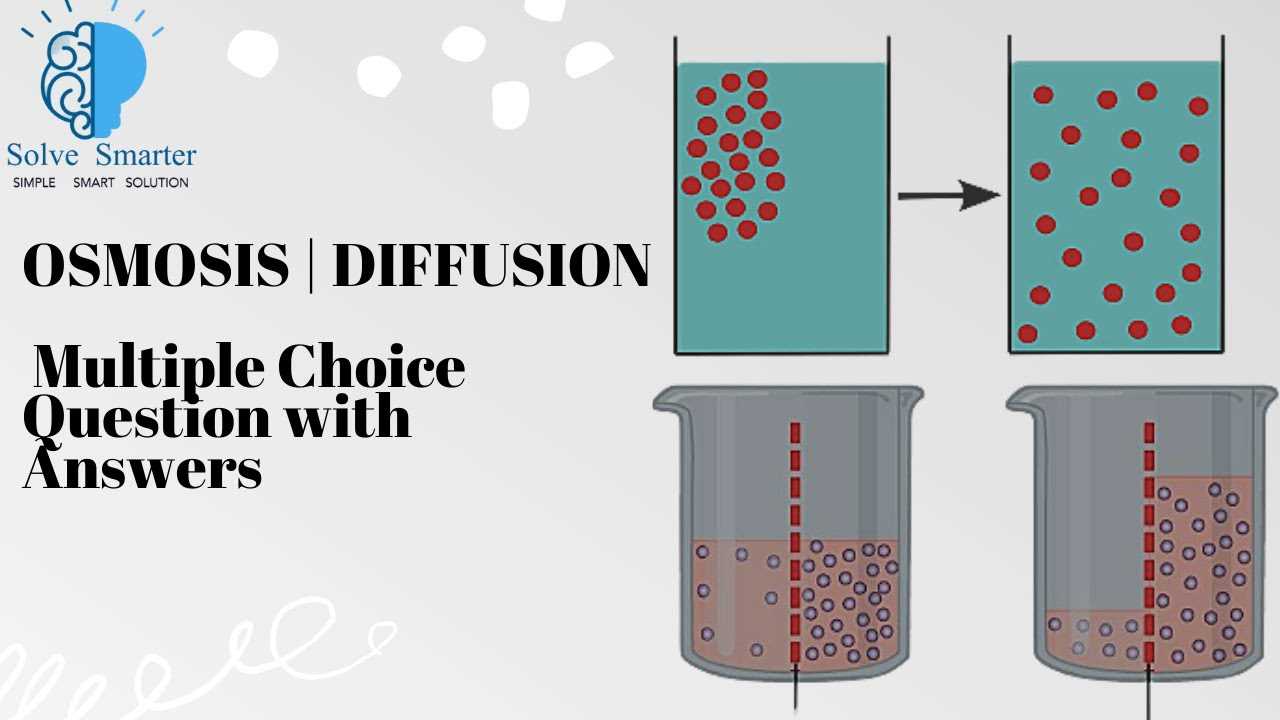
Diffusion vs Osmosis: Key Differences
Both diffusion and the movement of water across membranes are vital biological processes that allow substances to move between different areas. While these processes may seem similar, they have distinct characteristics and mechanisms. Understanding the differences between them is crucial for comprehending how cells maintain homeostasis and manage internal environments.
The primary distinction lies in the types of substances that move and the way they cross membranes. Diffusion involves the movement of molecules from areas of higher concentration to lower concentration, affecting gases and small molecules, whereas the movement of water through a selectively permeable membrane occurs under different principles, often driven by differences in pressure and solute concentration.
| Aspect | Diffusion | Water Movement |
|---|---|---|
| Substances Involved | Gases, small molecules | Water |
| Type of Membrane | No membrane needed | Requires selectively permeable membrane |
| Energy Requirement | Passive (no energy required) | Passive (no energy required) |
| Driving Force | Concentration gradient | Concentration gradient and pressure |
Understanding Osmosis in Living Cells
The movement of water and solutes through cell membranes is a fundamental process that supports many vital functions within living organisms. This movement helps cells maintain their shape, regulate their internal environment, and ensure that necessary nutrients and waste products are properly balanced. In living cells, this mechanism plays an essential role in sustaining life and supporting cellular activities.
Role of Water Movement

Water is the most important substance involved in this process. Its movement across the cell membrane helps maintain cellular structure and function. In plant cells, for example, water creates internal pressure, or turgor, which keeps the cells rigid and supports the plant’s overall structure. In animal cells, the careful regulation of water flow ensures that cells neither shrink nor swell uncontrollably, which could lead to cell damage.
Impact on Cellular Functions
The ability of cells to control the movement of water allows them to perform essential functions such as nutrient absorption, waste removal, and maintaining cell volume. Any disruption in this balance can result in serious health consequences, such as dehydration, swelling, or the inability to properly regulate internal environments.
Factors Affecting Osmosis Rates
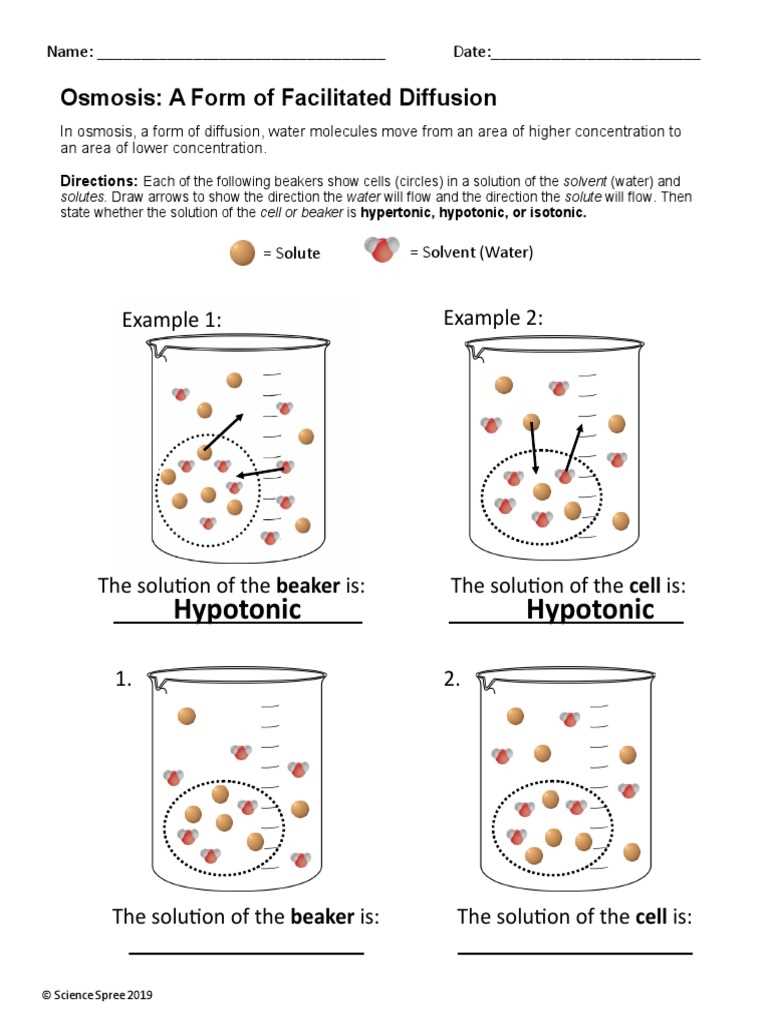
The rate at which substances move across a membrane is influenced by various factors that can either speed up or slow down the process. Understanding these factors is essential for grasping how cells maintain balance and function properly. Changes in environmental conditions, the properties of the membrane, and the nature of the substances involved can all affect how quickly molecules move from one side of the membrane to the other.
One of the most significant factors is the concentration gradient. A steeper difference in concentration between two areas will generally result in a faster rate of movement as molecules naturally move toward areas of lower concentration. Other factors, such as temperature and pressure, can also play a crucial role. Higher temperatures can increase the kinetic energy of molecules, leading to faster movement, while increased pressure can push molecules through membranes more quickly.
Additionally, the permeability of the membrane itself is a key determinant. Membranes that are more permeable to certain substances will allow them to pass through more easily, whereas more selective or less permeable membranes will slow down the movement of molecules. The size, charge, and solubility of the molecules involved also impact their ability to cross the membrane efficiently.
Osmosis in Plant Cells Explained
In plant cells, the movement of water plays a crucial role in maintaining structure, supporting growth, and enabling the transportation of essential nutrients. The process of water movement through plant cells is fundamental to how plants stay turgid, allowing them to remain upright and absorb sunlight efficiently. The unique properties of plant cell walls and vacuoles help regulate this movement, ensuring the plant’s overall health and stability.
How Water Moves in Plant Cells
Water flows through plant cells in response to differences in solute concentration inside and outside the cell. Here are the key steps:
- Water Uptake: Water enters the plant cell from the surrounding environment, moving through the cell membrane and into the vacuole.
- Cell Wall Interaction: The rigid cell wall provides structural support, preventing the cell from bursting under pressure while allowing water to enter freely.
- Maintaining Turgidity: The vacuole fills with water, creating internal pressure (turgor pressure) that helps keep the plant cell firm and functional.
Effects of Water Movement
The proper movement of water is vital for plant cells to perform key functions such as nutrient transport, waste removal, and photosynthesis. When water moves into plant cells, it supports cell expansion and growth. If water is lost or not absorbed efficiently, plant cells can shrink and lose their structural integrity, which can lead to wilting or even death of the plant.
How Osmosis Influences Water Movement
Water movement within biological systems is driven by various factors that regulate how fluids travel across different environments. The movement of water, particularly through membranes, is a fundamental process that supports cellular activities and overall organism health. This movement is influenced by the concentration of solutes and the pressure differences between various compartments, leading to essential changes in how water is distributed within cells and tissues.
Role of Concentration Gradient
Water naturally moves from areas of lower solute concentration to areas of higher concentration, a process that helps balance the internal environment of cells. This movement is crucial for maintaining cellular hydration and nutrient exchange. When cells encounter a higher concentration of solutes, they draw in water to maintain equilibrium, which can affect their size and function.
Impact of Pressure on Water Flow
In addition to concentration differences, pressure plays a significant role in how water moves through cells. Increased pressure can push water into cells, while decreased pressure can lead to water loss. In plants, this is especially important as pressure within plant cells supports turgidity, allowing them to remain firm and upright. Without this pressure, plant cells would become limp, and the plant could lose its structural integrity.
Real-Life Applications of Osmosis
The movement of water through different environments is not only a fundamental biological process but also plays a crucial role in a wide range of real-life applications. From preserving food to treating medical conditions, the principles behind fluid movement across membranes are applied in numerous fields. Understanding how water behaves in various contexts can help solve practical problems and improve technologies that affect our daily lives.
Food Preservation and Cooking
One of the most common applications of water movement is in food preservation. By controlling the movement of water into or out of food items, we can influence their texture, shelf life, and taste. Some examples include:
- Salt and sugar curing: The high concentration of salt or sugar in certain foods draws water out of microbial cells, inhibiting bacterial growth and prolonging freshness.
- Pickling: The osmotic movement of water into vegetables helps preserve them while also infusing them with flavors.
Medical Treatments
In healthcare, the understanding of how fluids move through the body can be crucial for treating various conditions. Key examples include:
- Dialysis: This medical procedure relies on the principle of fluid movement through membranes to filter waste products from the blood in patients with kidney failure.
- IV fluid administration: Proper balance of fluids and electrolytes is essential for patient hydration and recovery. The rate at which fluids are absorbed can be adjusted to meet the specific needs of the patient.
Osmosis and Cell Turgidity in Plants
In plants, maintaining proper water balance is essential for structural integrity and growth. The way water enters and moves within plant cells plays a key role in ensuring that they stay firm and functional. This water movement creates internal pressure within the cells, which helps them maintain their shape and resist external forces. Without this process, plants would lose their rigidity and ability to stand upright, impacting their ability to absorb sunlight and carry out photosynthesis.
When plant cells take in water, the pressure inside increases, causing the cell to swell. This internal pressure, known as turgor pressure, is critical for supporting the plant’s overall structure. If the cells do not have enough water, the turgor pressure drops, leading to wilting or even the death of the plant. Conversely, when there is too much water, cells can become turgid, but the cell wall prevents them from bursting, allowing plants to maintain a balance between rigidity and flexibility.
Effects of Osmosis on Animal Cells
The movement of water in and out of animal cells is crucial for their survival and function. Unlike plant cells, which have a rigid cell wall to provide structure, animal cells rely on the balance of internal and external water concentrations to maintain their shape and prevent damage. The process that regulates this water movement is essential for maintaining cell volume, pressure, and overall health. If this balance is disrupted, it can lead to a variety of cellular problems.
When water enters or leaves a cell in an unregulated manner, it can cause the cell to swell or shrink. This phenomenon can have serious consequences:
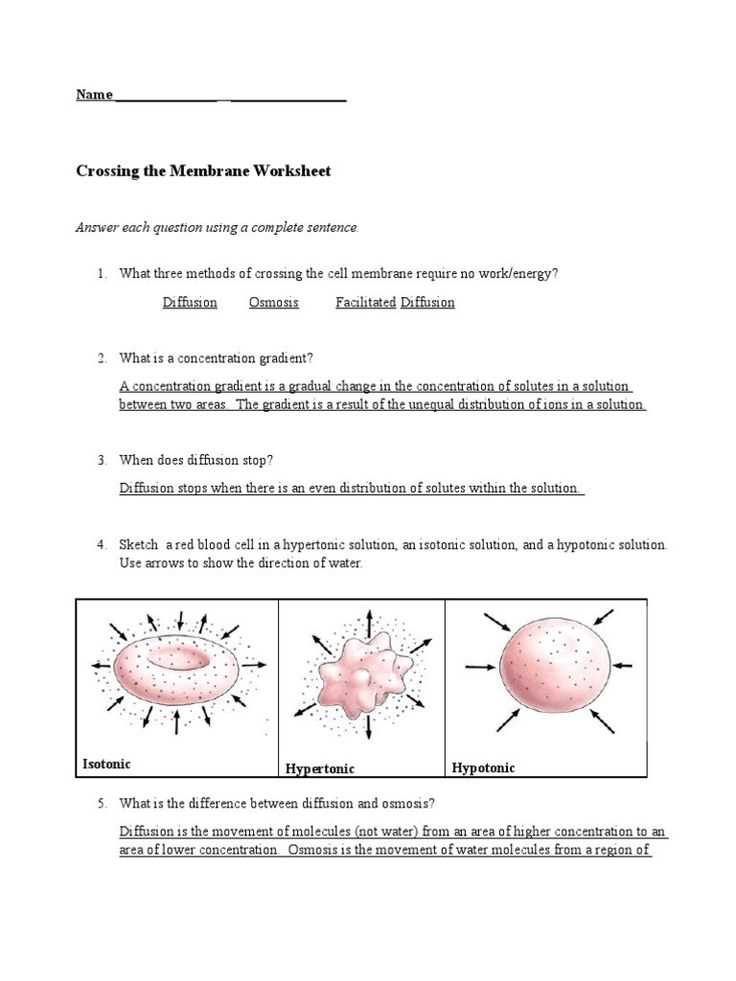
- Hypotonic solution: If animal cells are placed in a solution with a lower concentration of solutes than the cell’s interior, water flows into the cells. This can cause them to swell and possibly burst, a process known as lysis.
- Hypertonic solution: In a solution with a higher concentration of solutes, water moves out of the cells, leading to shrinkage. This can impair cell function and lead to dehydration.
Common Osmosis Experiment Results
In laboratory settings, various experiments are conducted to observe how water moves across cell membranes under different conditions. These experiments provide valuable insights into how cells interact with their environment and how they maintain homeostasis. By manipulating factors like concentration gradients, researchers can observe the effects of water movement, which leads to clear outcomes that highlight the principles of passive transport.
Typical Outcomes of Experiments
In most experiments, the results can be grouped into a few key observations, depending on the type of solution used:
- Swelling of Cells: When cells are placed in a solution with lower solute concentration than the cell’s interior, water moves into the cell. This can cause the cells to swell, as they absorb water to balance the concentration difference.
- Shrinkage of Cells: When cells are exposed to a higher concentration of solutes, water flows out of the cells, leading to shrinkage. This is commonly observed in plant or animal cells placed in hypertonic solutions.
Real-World Implications
These experiments help demonstrate how cells manage water balance in real-world environments, from plant root systems absorbing water from soil to animal cells maintaining fluid balance in the body. Understanding these results is crucial in fields like medicine, agriculture, and biotechnology.
How Osmosis Relates to Homeostasis
Water regulation within living organisms plays a crucial role in maintaining a stable internal environment, often referred to as homeostasis. The movement of water across cell membranes is central to this process, as cells must balance the intake and loss of water to function properly. When the balance of water inside and outside the cells is disrupted, it can lead to conditions that affect the overall health of the organism.
The Role of Water Movement
In order to maintain homeostasis, cells rely on the natural movement of water. This process ensures that cells do not become too swollen or dehydrated, which could disrupt cellular functions. Key factors like solute concentration and membrane permeability dictate how water moves into or out of the cell, helping to regulate its internal environment.
Maintaining Balance in Cells
Various systems within an organism work together to maintain this balance. For example, the kidneys in animals regulate the amount of water and solutes in the bloodstream, while plants manage water through their root systems. These mechanisms ensure that water levels stay within the necessary range for the cells to carry out essential processes.
| Scenario | Effect on Homeostasis |
|---|---|
| High Solute Concentration Outside the Cell | Water moves out of the cell, leading to dehydration and potential cell shrinkage. |
| Low Solute Concentration Outside the Cell | Water moves into the cell, which may cause swelling, but helps maintain hydration. |
By regulating the movement of water in and out of cells, organisms ensure that homeostasis is maintained, allowing cells to perform their vital functions and keep the organism in a stable state.
Answering Common Osmosis Questions
Many questions arise when discussing the movement of water across cell membranes, particularly in relation to how it affects living organisms. This section aims to address the most frequently asked questions, offering clarity and insight into how this process works in various biological systems.
What is the Direction of Water Movement?
Water naturally moves from areas of low solute concentration to areas of high solute concentration. This is due to the tendency of water molecules to balance concentrations across a membrane. The process continues until equilibrium is reached, meaning the concentrations on both sides of the membrane are equal.
What Happens When Cells Lose Too Much Water?
If a cell loses too much water, it may begin to shrink and lose its functionality. This is known as plasmolysis in plant cells or crenation in animal cells. Both scenarios can be harmful, as the loss of water disrupts the normal cell processes.
- In plant cells, loss of water leads to reduced turgidity, making the plant less rigid.
- In animal cells, dehydration can cause a breakdown in metabolic functions.
Why Do Cells Need Water?
Water is essential for maintaining the structural integrity of cells and for facilitating biochemical reactions. It helps transport nutrients and waste products, aids in temperature regulation, and supports the overall function of the organism. Without proper water balance, cellular processes can be impaired.
Can Osmosis Occur Without a Semi-Permeable Membrane?
No, a semi-permeable membrane is essential for this process. The membrane allows water molecules to pass through while restricting the movement of certain solutes. Without this selective barrier, the process would not be able to control the movement of water in the desired direction.
These common questions provide a better understanding of how water movement works within biological systems. By answering these fundamental inquiries, we can gain a clearer picture of the importance of water regulation in sustaining life.