
Understanding the flow of energy during chemical processes is essential for anyone studying the physical sciences. This area of study focuses on how heat is transferred and how it influences the direction and speed of reactions. A solid grasp of these concepts allows you to predict reaction behaviors and calculate the energy involved in various chemical transformations.
Throughout this guide, we explore essential topics that test your knowledge of energy shifts, including the calculations and principles behind them. By familiarizing yourself with key formulas and laws, you’ll be able to tackle a wide range of related tasks with confidence. Whether you’re dealing with heat release or absorption, the ability to quantify these changes is fundamental for mastering the subject.
In the upcoming sections, we will break down complex ideas into more manageable parts, providing clear explanations and examples to ensure a deep understanding. From the laws governing energy conservation to practical applications in real-world scenarios, each section will guide you through critical concepts, helping you prepare effectively for any related challenges.
Thermochemistry Exam Questions and Answers
Mastering the flow of energy during chemical processes involves more than just memorizing formulas; it requires a deep understanding of how energy changes impact reactions. This section is designed to help you apply your knowledge to different scenarios, testing your ability to solve problems related to heat transfer and energy conservation. By reviewing a variety of example tasks and their solutions, you will become better equipped to tackle challenges in this field.
Each problem in this section is carefully chosen to reflect the types of situations you may encounter when evaluating energy shifts in chemical reactions. From calculating heat exchanged in a reaction to understanding the relationship between bond energies and reaction outcomes, these tasks offer a comprehensive review of essential concepts. By working through these examples, you’ll enhance your problem-solving skills and be able to approach similar challenges with greater ease.
While solving these problems, it’s crucial to focus on the underlying principles that guide energy changes. Understanding how to apply laws like the conservation of energy, as well as recognizing the differences between endothermic and exothermic processes, will allow you to confidently work through similar exercises in the future. This section serves as both a review and a guide to help you refine your skills in applying theoretical knowledge to practical situations.
Understanding Thermochemistry Concepts
To fully grasp the principles of energy flow during chemical reactions, it’s important to first understand the core concepts that govern how heat interacts with matter. These principles provide the foundation for analyzing and predicting how substances exchange energy during transformations. The study of these interactions reveals patterns that are essential for both theoretical and practical applications in science and engineering.
Central to this understanding is the idea that energy cannot be created or destroyed, but can only be transformed from one form to another. This principle, along with the concepts of enthalpy, internal energy, and heat capacity, forms the basis for calculating and predicting how much heat is absorbed or released in a reaction. By exploring these concepts in detail, you can gain insight into the underlying mechanisms that drive chemical changes and influence reaction outcomes.
As you explore the concepts further, it’s crucial to recognize the distinction between different types of energy changes. For example, reactions can either absorb heat from the surroundings or release it, which directly affects the products and energy balance of the system. Understanding these differences allows for more accurate predictions and better problem-solving when applying these ideas in real-world situations.
Key Principles of Energy in Reactions
The behavior of energy during chemical processes is governed by several key principles that dictate how energy is transferred or transformed in the system. Understanding these principles is crucial for predicting the outcome of reactions and solving related problems. These concepts provide insight into how heat is absorbed or released and the factors that influence energy changes within chemical systems.
- Conservation of Energy: Energy cannot be created or destroyed, only converted from one form to another. This fundamental law ensures that the total energy of the system and its surroundings remains constant.
- Enthalpy: This refers to the total heat content of a system, which includes both internal energy and the work done by the system. It is a key factor in understanding the heat exchange during a reaction.
- Endothermic and Exothermic Reactions: Reactions can either absorb heat from the surroundings (endothermic) or release heat into the surroundings (exothermic), depending on the energy change that occurs during the process.
- Heat Capacity: This is the amount of heat required to raise the temperature of a substance by one degree. It plays a vital role in understanding how much energy is needed to cause a temperature change.
These principles are interconnected and must be considered together when analyzing energy changes in reactions. Understanding how each principle applies to different types of processes allows for a more comprehensive understanding of how energy moves within chemical systems. By mastering these concepts, you can confidently approach calculations and predictions related to energy shifts in reactions.
Enthalpy and Its Role in Chemical Reactions

Enthalpy plays a central role in understanding the heat exchange during chemical processes. It is a state function that helps describe the total heat content of a system, taking into account both internal energy and the work done by the system. By focusing on enthalpy, we can gain insight into how energy flows during reactions, whether heat is absorbed or released, and how this affects the overall outcome of a chemical transformation.
The change in enthalpy, often referred to as ΔH, is used to quantify the heat gained or lost in a reaction at constant pressure. This concept is particularly useful when analyzing reactions that occur in open systems, where pressure remains constant, such as in many laboratory settings. Whether a reaction is exothermic or endothermic, the change in enthalpy directly correlates with the amount of heat involved in the process.
Understanding enthalpy allows for more accurate predictions about the energy requirements or energy release in various chemical reactions. It also forms the basis for calculating other important thermodynamic properties, such as Gibbs free energy and spontaneity. Whether working with combustion reactions, phase changes, or solvation processes, enthalpy serves as a fundamental concept in determining the heat effects that accompany chemical transformations.
Common Thermochemical Calculations Explained

In the study of energy changes during chemical processes, performing accurate calculations is essential for predicting the heat exchanged in reactions. These calculations help quantify how much energy is absorbed or released, allowing for a deeper understanding of the underlying mechanisms. Here, we will explore some of the most common calculations involved in evaluating energy shifts in chemical reactions.
Heat Calculations Using Specific Heat Capacity

One of the fundamental calculations involves determining the heat absorbed or released by a substance during a temperature change. This can be calculated using the formula:
q = mcΔT
Where q is the heat absorbed (in joules), m is the mass of the substance, c is the specific heat capacity, and ΔT is the change in temperature. This calculation is crucial for determining the heat involved when substances undergo temperature changes, such as in calorimetry experiments.
Enthalpy Change in Reactions
Another common calculation is determining the enthalpy change for a given reaction. The change in enthalpy (ΔH) can be calculated by using the following equation:
ΔH = ΣH(products) – ΣH(reactants)
In this case, the enthalpy change is the difference between the enthalpy of the products and the enthalpy of the reactants. This formula is often used to calculate the heat released or absorbed during a chemical reaction at constant pressure, helping to identify whether the process is exothermic or endothermic.
By mastering these and other related calculations, you can efficiently determine the energy changes in various chemical reactions and make accurate predictions about their outcomes.
Heat Transfer Methods in Chemical Reactions
Understanding how heat moves within a system is crucial for analyzing the energy changes that occur during chemical reactions. Heat can be transferred in various ways, depending on the nature of the system and the reaction. These methods of heat transfer play a significant role in determining how substances absorb or release energy during transformations, and they help us predict the behavior of the system in different conditions.
Conduction
Conduction is the transfer of heat through direct contact between molecules. In this process, heat flows from the warmer part of the substance to the cooler part, as molecules with higher kinetic energy collide with those with lower energy. This method is particularly relevant in solid materials, where particles are closely packed, making it easier for energy to be transferred between them. Understanding conduction is important when studying reactions occurring in solid phases or in systems where temperature gradients are present.
Convection
Convection refers to the transfer of heat through the movement of fluids, such as liquids or gases. In this process, warmer areas of the fluid rise due to their lower density, while cooler areas sink, creating a circulation that facilitates heat transfer. Convection is a key factor in reactions that take place in solutions or gaseous environments, where heat needs to be transported through bulk movement of the substance rather than molecular collisions.
Both conduction and convection are essential in understanding the dynamics of energy flow during chemical reactions. By mastering these heat transfer methods, it becomes possible to predict how temperature changes will influence reaction rates, product formation, and other key aspects of chemical processes.
Applications of Hess’s Law in Assessments
Hess’s Law is a powerful tool for calculating the heat changes involved in chemical reactions, especially when direct measurement is not feasible. It states that the total enthalpy change of a reaction is the sum of the enthalpy changes of the steps into which the reaction can be divided. This principle is widely applied in various problem-solving scenarios where multiple reactions are involved, allowing for the determination of enthalpy changes indirectly.
In assessments, you are often required to apply Hess’s Law to calculate the overall enthalpy change for a reaction by using known enthalpy values of other related reactions. This approach eliminates the need for direct experimentation and enables the prediction of heat changes in complex reactions that occur in multiple stages. By manipulating the given reactions and their corresponding enthalpies, you can piece together the solution to a more complex problem.
One common type of problem involves using Hess’s Law to combine reactions and find the enthalpy change for a target reaction. By reversing or scaling the reactions as needed, you can apply the law to determine the heat of a reaction even if it occurs in several steps. Mastering this concept will not only improve your problem-solving skills but also help you work efficiently with thermodynamic data.
Bond Energies and Reaction Predictability
The energy required to break and form chemical bonds plays a crucial role in determining whether a reaction will proceed spontaneously or require external energy input. By understanding bond energies, it becomes possible to predict the overall energy changes in a reaction, helping to assess whether it will release or absorb energy. This principle is essential for evaluating reaction feasibility and understanding the underlying energy shifts that drive chemical transformations.
Bond energies represent the strength of the bonds between atoms in a molecule. When bonds are broken, energy is absorbed, and when new bonds are formed, energy is released. The difference between these energy changes dictates the net energy flow during a reaction. If more energy is released than absorbed, the reaction will typically be exothermic, whereas if more energy is required to break bonds than is released by the formation of new ones, the reaction will be endothermic.
By calculating the bond energies of reactants and products, it’s possible to predict the direction of energy flow in a reaction. This knowledge is particularly useful in determining reaction conditions and optimizing processes in both laboratory and industrial settings. By mastering the concept of bond energies, one can make accurate predictions about the energy dynamics of chemical reactions and the likelihood of their occurrence under certain conditions.
Calorimetry: Techniques and Questions
Calorimetry is a technique used to measure the heat released or absorbed during a chemical or physical process. This method is essential for understanding the energy changes that occur in reactions, and it provides valuable insights into reaction mechanisms and thermodynamic properties. By using various calorimetric methods, it becomes possible to quantify energy flow and make predictions about reaction behavior under different conditions.
Common Calorimetric Methods
There are several techniques employed in calorimetry, each suited to different types of reactions and systems. Some of the most common methods include:
- Bomb Calorimetry: Used for reactions that occur at constant volume, typically combustion reactions, to measure the heat released.
- Coffee Cup Calorimetry: A simpler, less expensive method for measuring heat changes in reactions occurring at constant pressure.
- Adiabatic Calorimetry: Measures the heat flow in an insulated system to assess the heat released or absorbed by a process without heat exchange with the surroundings.
Common Problems in Calorimetric Calculations
While calorimetry provides important data, interpreting this data correctly requires a solid understanding of the underlying principles. Common challenges in calorimetric calculations include:
- Heat Capacity Determination: Accurate measurements of the heat capacity of the system are essential for correct calculations. Misestimations can lead to incorrect results.
- Heat Loss: In many calorimetric experiments, some heat may be lost to the surroundings, which must be accounted for to ensure precision in the measurements.
- Reaction Completeness: Not all reactions proceed to completion. Incomplete reactions can affect the accuracy of heat measurement.
Mastering the techniques of calorimetry and understanding how to handle common issues in heat measurement are critical for anyone studying energy changes in chemical reactions. By applying the right methods and addressing common pitfalls, it is possible to gather reliable data that can be used for further analysis and application.
Standard State and Its Significance
The concept of standard state plays a fundamental role in calculating thermodynamic properties and determining reaction enthalpies. It refers to the reference conditions under which substances are most commonly studied, allowing for consistent comparisons and calculations across different chemical systems. By defining a standard state, it becomes possible to evaluate the energy changes in a uniform manner, regardless of the form or phase of the substance involved in a particular process.
Defining Standard State

A substance’s standard state is typically defined as its most stable form at a specified temperature, usually 298 K (25°C), and 1 atmosphere of pressure. For example, the standard state of oxygen is the diatomic molecule (O₂) in the gas phase, while the standard state of water is liquid at these conditions. This reference allows for a uniform basis when measuring enthalpy, entropy, and Gibbs free energy.
Importance in Thermodynamic Calculations
The standard state is crucial in thermodynamic calculations as it provides a baseline for calculating changes in enthalpy, entropy, and Gibbs free energy. By referencing substances in their standard states, one can more accurately predict reaction behavior and energy changes. Additionally, the concept of standard enthalpy of formation, which refers to the heat change when one mole of a compound is formed from its elements in their standard states, is key to understanding reaction energetics and predicting outcomes.
Without the use of a standard reference, calculating the energy changes of reactions would become inconsistent and unreliable. The standard state provides a reliable framework for comparison, ensuring that data can be applied universally across various chemical processes.
Factors Influencing Heat of Reactions
The heat released or absorbed during a chemical reaction is influenced by several factors, each playing a significant role in determining the overall energy change. Understanding these factors is essential for predicting the thermal behavior of reactions under various conditions. These variables include the nature of the reactants, the reaction conditions, and the pathway taken during the transformation. By analyzing these factors, it becomes possible to better understand the energetic dynamics of chemical processes.
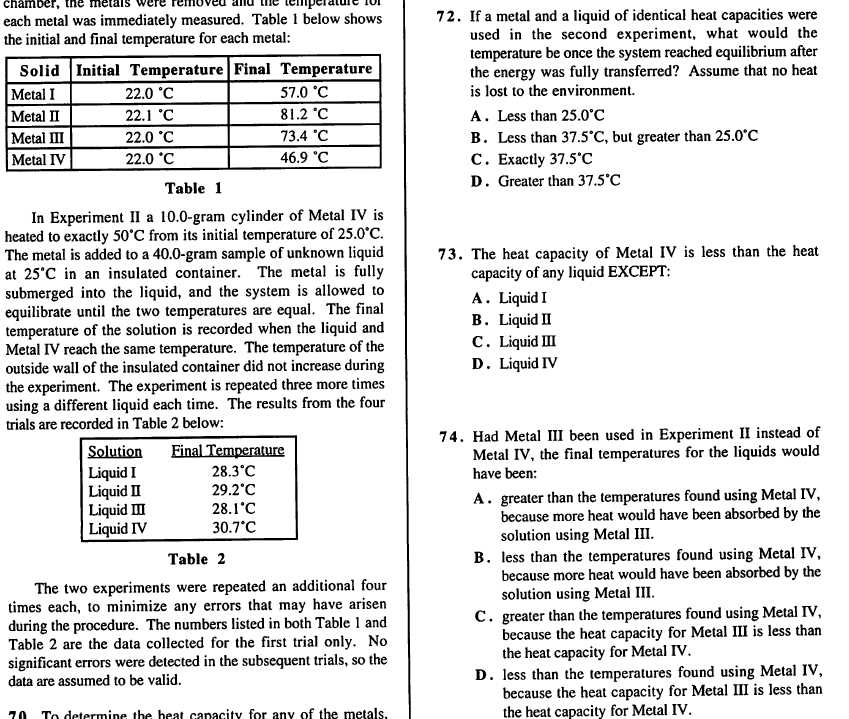
One of the primary factors influencing the heat of a reaction is the strength of the chemical bonds involved. Reactions that break stronger bonds require more energy to initiate but often release more energy when new, stronger bonds are formed. In contrast, reactions involving weaker bonds may release less energy. The difference in bond energies between reactants and products directly impacts the overall heat of the reaction.
Temperature and pressure are also critical factors. At higher temperatures, the kinetic energy of molecules increases, which can lead to faster reactions and potentially more heat production. Pressure, particularly in reactions involving gases, can alter the volume and concentration of reactants, affecting the reaction rate and the heat exchanged. Additionally, the phase of the substances involved can influence how heat is absorbed or released during the process.
Another important factor is the reaction pathway itself. Some reactions occur in a single step, while others involve multiple stages or intermediate compounds. The energy required to reach the final product can vary depending on whether the reaction occurs directly or through several intermediate steps. Each stage in the reaction can contribute differently to the overall heat change.
By considering all these factors, it is possible to predict and manipulate the heat changes during chemical reactions, which is essential in both laboratory research and industrial applications.
Important Formulas for Energy Calculations
Understanding the key formulas involved in energy calculations is essential for accurately determining the heat flow and energetic changes in chemical processes. These formulas help quantify the heat released or absorbed during reactions and provide valuable insights into reaction dynamics. Below are some of the fundamental equations used to calculate energy changes and thermodynamic properties in chemical reactions.
| Formula | Explanation |
|---|---|
| q = m × C × ΔT | Calculates the heat absorbed or released by a substance, where q is the heat energy, m is the mass of the substance, C is its specific heat capacity, and ΔT is the temperature change. |
| ΔH = ΣH(products) – ΣH(reactants) | This equation represents the enthalpy change of a reaction, calculated by subtracting the total enthalpy of the reactants from that of the products. |
| ΔG = ΔH – TΔS | The Gibbs free energy equation, which helps determine the spontaneity of a reaction. ΔH is the enthalpy change, ΔS is the entropy change, and T is the absolute temperature. |
| ΔH = Σ(ΔHf(products)) – Σ(ΔHf(reactants)) | This formula is used to calculate the enthalpy change of a reaction using the standard enthalpy of formation values (ΔHf) for the products and reactants. |
| q = -m × C × ΔT | Used in calorimetry to measure heat exchanged, where q is the heat energy, m is the mass of the sample, C is its specific heat capacity, and ΔT is the change in temperature. The negative sign indicates heat loss by the system. |
By mastering these formulas, it becomes easier to predict reaction behavior, calculate energy changes, and analyze how energy flows within chemical systems. These equations form the foundation for studying the energetic aspects of reactions and provide a reliable method for calculating heat exchange and thermodynamic properties.
Exothermic vs Endothermic Reactions

Chemical reactions can either release or absorb energy in the form of heat, depending on the nature of the process. The distinction between these two types of reactions plays a crucial role in understanding how energy flows in a system. Reactions that release energy are fundamentally different from those that require energy to proceed. This section explores the key differences between these two reaction types and how they impact the surrounding environment.
Exothermic Reactions
Exothermic reactions are processes that release heat into their surroundings. This energy release typically results in an increase in temperature of the surrounding environment. During these reactions, the products formed are at a lower energy state than the reactants, meaning that excess energy is released as heat. Common examples of exothermic reactions include combustion, such as the burning of fuel, and the formation of ionic compounds from their constituent elements.
In an exothermic reaction, the total energy required to break the bonds in the reactants is less than the energy released when the new bonds in the products are formed. As a result, the overall enthalpy change (ΔH) is negative, indicating that energy is released. This release of heat is often harnessed for practical purposes, such as in heat engines or chemical heating pads.
Endothermic Reactions
Endothermic reactions, on the other hand, absorb heat from their surroundings. These reactions require an input of energy to proceed, which leads to a decrease in the temperature of the environment. In these processes, the products formed are at a higher energy state compared to the reactants. This energy is absorbed to break the bonds in the reactants, and the reaction cannot proceed without this energy input.
In an endothermic reaction, the energy required to break the bonds in the reactants exceeds the energy released when the products are formed. Consequently, the enthalpy change (ΔH) is positive, indicating that energy is absorbed. Examples of endothermic reactions include the dissolution of certain salts in water and the process of photosynthesis in plants.
Understanding the differences between exothermic and endothermic reactions is essential for predicting reaction behavior and managing energy in various applications. Both types of reactions have significant roles in natural processes and industrial applications, with each offering distinct challenges and benefits depending on the context.
Real-World Applications of Thermochemistry
The principles of energy changes during chemical reactions are crucial in various practical applications across many industries. Understanding how energy is transferred during these reactions allows us to design more efficient processes, optimize energy use, and harness reactions for specific purposes. From the creation of energy sources to the development of materials, energy transformations play a key role in our daily lives and technological advancements.
Energy Production
One of the most significant real-world applications involves the generation of energy. Many industries rely on energy-releasing reactions to power machinery, vehicles, and even entire cities. Some common examples include:
- Combustion of Fossil Fuels: The burning of coal, oil, and natural gas generates large amounts of energy in the form of heat, which is used to produce electricity.
- Nuclear Power: Nuclear reactions, which release energy from atomic nuclei, are harnessed in power plants to generate electricity on a massive scale.
- Renewable Energy: Reactions involved in bioenergy, wind, and solar technologies convert natural sources of energy into usable electricity or fuel.
Food and Cooking
The principles of heat and energy transfer are also central to cooking and food processing. Here, energy is transferred during chemical reactions that alter the properties of food, such as:
- Exothermic Reactions in Baking: The release of energy when baking ingredients like flour and sugar interact at high temperatures creates the texture and flavor of baked goods.
- Endothermic Reactions in Cooking: The absorption of heat during boiling or frying causes food to change in texture, color, and taste.
Industrial Manufacturing

In the manufacturing sector, understanding energy changes is key to optimizing production processes and improving material properties. Some notable applications include:
- Chemical Manufacturing: Reactions used to create industrial chemicals, plastics, and synthetic materials often require precise control of temperature and energy input.
- Metal Production: The extraction of metals from ores involves energy-releasing reactions, such as the reduction of ores in furnaces.
- Pharmaceuticals: Chemical reactions that produce medicinal compounds depend on specific temperature and energy conditions to ensure purity and efficacy.
Environmental Impact
Environmental management also benefits from the application of energy principles. By understanding and controlling energy changes, we can minimize harmful emissions and improve sustainability:
- Carbon Capture: Reactions designed to capture and store carbon dioxide can help mitigate the impact of fossil fuel consumption on the atmosphere.
- Waste Management: Chemical processes that break down waste materials often rely on energy absorption or release to efficiently manage waste and reduce landfill use.
These examples illustrate just a few ways that the principles of energy changes in reactions shape industries, enhance technologies, and address environmental challenges. The practical uses of these concepts extend far beyond the laboratory, making them integral to the progress of modern society.
Study Tips for Thermochemistry Exam
Preparing for assessments that cover the principles of energy changes in chemical reactions requires a strategic approach to ensure understanding and retention. By focusing on key concepts, practicing with relevant problems, and organizing your study routine, you can improve your performance and tackle even the most challenging topics. This section offers some practical tips to guide your study sessions effectively.
1. Understand the Fundamental Concepts

Before diving into problem-solving, it’s essential to have a solid understanding of the basic principles. These include energy transfer, enthalpy, heat capacities, and types of reactions. Make sure to grasp the core ideas before attempting complex calculations or concepts.
2. Break Down Complex Topics
Some concepts may seem overwhelming at first, especially when they involve intricate formulas or abstract principles. Break these down into smaller, manageable parts. Focus on one concept at a time and use visual aids, like diagrams or flowcharts, to simplify your understanding.
3. Practice Regularly with Sample Problems
Hands-on practice is one of the most effective ways to reinforce your knowledge. Work through sample problems that cover a range of topics, including heat flow, specific heat calculations, and energy transformations. The more problems you solve, the more confident you will become in applying the concepts.
4. Create a Study Schedule
Developing a study schedule is crucial for consistent progress. Allocate specific times for studying different topics, and stick to your plan. Make sure to review previous material regularly to reinforce your knowledge and avoid cramming at the last minute.
5. Use a Variety of Resources
In addition to your textbooks, consider using online tutorials, videos, and other study materials to broaden your understanding. Many websites and YouTube channels offer clear explanations and real-life examples that can make complex ideas easier to grasp.
6. Form a Study Group
Collaborating with peers can be a valuable study technique. Discussing concepts and solving problems together can help clarify difficult topics and expose you to different perspectives. Group study can also motivate you to stay on track and engage in active learning.
7. Test Yourself
Self-testing is a great way to assess your knowledge and pinpoint areas that need improvement. Use past practice problems, quizzes, or mock tests to simulate the conditions of an actual assessment. This helps reduce test anxiety and boosts your confidence.
8. Focus on Key Formulas and Calculations
Ensure that you are familiar with essential formulas and know how to apply them accurately. Pay close attention to key equations, such as those for calculating heat transfer, and practice manipulating them in different scenarios.
9. Stay Organized and Take Notes
Good organization can significantly enhance your study efficiency. Keep a notebook where you write down important formulas, definitions, and concepts. Use different colors or bullet points to organize information clearly. Reviewing these notes before the assessment can be incredibly helpful.
10. Stay Calm and Confident
Finally, maintaining a calm and confident mindset is key to performing well. Avoid overloading yourself with information or stressing out. Stay positive, take breaks when needed, and ensure you get adequate rest before the assessment.
| Study Tip | Benefits |
|---|---|
| Understand the Fundamentals | Provides a strong foundation for tackling more complex topics |
| Break Down Complex Topics | Makes challenging material more manageable and easier to comprehend |
| Practice Regularly | Reinforces knowledge and improves problem-solving skills |
| Create a Study Schedule | Ensures steady progress and reduces last-minute stress |
| Use a Variety of Resources | Enhances understanding with different explanations and examples |
By following these tips and maintaining a disciplined study routine, you’ll be better prepared to handle the challenges presented in the assessment and apply the concepts with confidence.
How to Tackle Thermochemistry Problem Sets
Approaching a set of problems that involves energy changes in chemical reactions requires careful planning and systematic problem-solving techniques. Understanding the core concepts and applying them step by step is key to success. Below are strategies to effectively solve these types of problems and avoid common pitfalls.
1. Read the Problem Carefully
The first step in solving any problem is to thoroughly read the question. Take note of what is being asked and identify the known and unknown variables. Often, a problem will contain extra information meant to distract you, so focus on the key details.
2. Identify the Type of Calculation
These types of problems often involve specific calculations, such as heat transfer, energy changes, or enthalpy. Recognize the type of calculation required so you can apply the correct formula. Common examples include:
- Heat Transfer: Determine the amount of energy released or absorbed during a reaction.
- Enthalpy Changes: Calculate the difference in energy between reactants and products.
- Specific Heat Capacity: Find how much energy is needed to change the temperature of a substance.
3. Write Down the Relevant Equations
Once you know what type of problem you’re dealing with, write down the relevant equations. This will act as a guide and prevent you from forgetting crucial steps. Ensure you’re familiar with the formulas used to calculate energy, temperature changes, and reaction enthalpies.
4. Break the Problem into Manageable Steps
Instead of attempting to solve everything at once, break the problem into smaller, manageable steps. Tackle each part one at a time. For example, if you’re calculating the heat change, first identify the mass, temperature change, and specific heat capacity. Then, apply the formula.
5. Plug in the Known Values
Substitute the given values into the equations carefully. Be mindful of the units, ensuring consistency throughout. If necessary, convert the units (for example, from grams to kilograms or Celsius to Kelvin) to match the units used in the equations.
6. Solve and Double-Check Your Work

Perform the calculations, and once you’ve arrived at a solution, double-check your work. Look over the units, check for arithmetic errors, and ensure that the answer makes sense logically. If possible, compare your solution with a reference or previous example to verify your approach.
7. Practice Consistently
Consistent practice is essential for mastering problem sets. The more problems you solve, the better you will understand the principles and improve your problem-solving skills. Try tackling problems with varying levels of complexity to ensure you’re well-prepared for any challenge.
8. Seek Help When Needed
If you encounter a particularly challenging problem, don’t hesitate to seek help. Discussing the problem with classmates, instructors, or using online resources can provide a new perspective and clarify any confusion.
9. Stay Organized and Manage Your Time
Effective time management is essential when working through problem sets. Allocate enough time to work on each problem and avoid rushing through them. Stay organized by noting your approach, solutions, and any areas of difficulty for future review.
10. Review and Reinforce Your Learning
After completing a set of problems, take some time to review the solutions. Identify areas where you struggled and revisit those concepts. Reinforcing your learning through review ensures that you retain the information for future applications.
By following these strategies, you’ll improve your ability to solve problems with greater accuracy and confidence. Whether you’re preparing for an assessment or just strengthening your understanding, a methodical approach is key to success in tackling complex challenges.
Resources for Further Study
To deepen your understanding of energy changes in chemical reactions, it’s essential to use a variety of resources. Whether you’re looking for textbooks, online platforms, or interactive tools, the right materials can enhance your grasp of key concepts and improve your problem-solving skills. Below are some valuable resources that will help you further explore and strengthen your knowledge in this field.
1. Textbooks and Reference Books
Books provide a comprehensive foundation for learning theoretical concepts and exploring advanced topics. Here are some highly recommended texts:
| Book Title | Author(s) | Overview |
|---|---|---|
| Principles of Chemical Thermodynamics | Herbert B. Callen | This book offers an in-depth exploration of thermodynamics principles, focusing on energy changes and state functions. |
| Physical Chemistry | Peter Atkins, Julio de Paula | A comprehensive guide to various topics in physical chemistry, including energy transfer and reaction spontaneity. |
| Chemical Thermodynamics: Principles and Applications | J. Bevan Ott, Juliana B. Kesson | A detailed reference work for those interested in the practical applications of energy concepts in chemical reactions. |
2. Online Learning Platforms
Interactive online platforms offer courses, tutorials, and practice problems that can enhance your learning experience. Some of the top platforms include:
- Khan Academy – Free, self-paced courses with video lectures and practice exercises on energy, enthalpy, and related topics.
- Coursera – Provides university-level courses from institutions like Stanford and MIT, focusing on topics such as chemical reactions and energy transformations.
- edX – Another excellent platform offering courses on thermodynamics, chemistry, and reaction dynamics from top universities worldwide.
3. Scientific Journals and Articles
Reading peer-reviewed journals can provide you with the latest research, breakthroughs, and in-depth studies on reaction dynamics and energy. Here are a few journals to consider:
- The Journal of Physical Chemistry – Publishes articles related to the physical aspects of chemical reactions, including energy transfer and reaction mechanisms.
- Energy & Fuels – Focuses on the application of thermodynamic principles to energy generation and conversion processes.
- Nature Chemistry – Features cutting-edge research in the field of chemistry, including energy changes and thermodynamic applications in various reactions.
4. Interactive Tools and Simulations

Interactive tools can help visualize complex reactions and energy changes. Some useful websites and software include:
- PhET Interactive Simulations – Offers free simulations on various chemistry topics, including heat and energy in chemical reactions.
- ChemCollective Virtual Labs – Provides virtual labs that allow you to conduct experiments and calculate energy changes without needing physical lab equipment.
- Wolfram Alpha – A computational tool that helps solve chemical equations and perform calculations related to energy changes in reactions.
5. Study Groups and Discussion Forums
Joining study groups and participating in discussion forums allows you to collaborate with others and clarify concepts. Some popular forums include:
- Reddit – Chemistry Community – A platform for chemistry enthusiasts and students to discuss various topics, including energy changes in reactions.
- Stack Exchange – Chemistry – A Q&A forum where students and professionals can ask questions and discuss thermodynamic concepts.
- Study Groups on Discord or Facebook – Online groups where students can share resources, ask questions, and collaborate on difficult topics.
By utilizing these resources, you can expand your understanding of the principles governing chemical reactions and energy changes, and gain a deeper insight into real-world applications. Whether you prefer textbooks, interactive learning, or community discussions, these tools will help solidify your knowledge and support your ongoing studies.