The behavior of atoms and molecules can drastically change when they gain or lose electrons. This shift often leads to the creation of particles that are electrically charged. These charged entities play a fundamental role in various chemical reactions and processes. Grasping the way these particles form and interact is essential to understanding a wide range of phenomena in chemistry and physics.
In certain scenarios, it’s crucial to identify and describe these particles accurately, especially in chemical equations. The ability to recognize the nature of these particles helps explain how substances combine, break apart, and react in various conditions. Whether it’s in acids, salts, or simple elemental changes, the presence of charged particles is central to much of the science behind molecular transformations.
By focusing on their structure and behavior, it becomes easier to predict and control reactions. Whether through simple experimentation or complex modeling, understanding how particles change charge can lead to deeper insights into matter and its properties. This is why learning how to describe them properly is an important skill for anyone working with chemistry or related fields.
Understanding the Concept of Ions
In the study of chemistry, many elements and compounds can become charged under certain conditions. This change occurs when particles gain or lose electrons, resulting in a shift in their overall charge. These charged particles, known for their involvement in a wide array of chemical reactions, are fundamental to understanding molecular behavior and interactions. Their presence plays a critical role in everything from simple salt formation to complex biological processes.
The Process of Gaining or Losing Electrons

The transformation of a neutral atom or molecule into a charged particle happens through the gain or loss of electrons. When an atom acquires one or more electrons, it becomes negatively charged. On the other hand, when it loses electrons, it becomes positively charged. This change alters its interactions with other particles, making the atom or molecule more reactive and able to form bonds with other species.
Significance in Chemical Reactions
The creation of charged particles is essential in many types of chemical reactions, including those that occur in acids, salts, and biological systems. These particles are often the key players in processes such as conductivity, neutralization, and electrolyte balance. For example, when salts dissolve in water, they break apart into their constituent charged particles, which are then free to interact with other substances. Understanding this behavior is critical for fields ranging from medicine to materials science.
What Does It Mean to Be an Ion
When a particle gains or loses electrons, it acquires an electrical charge, becoming a different entity compared to its neutral state. This transformation results in a particle that behaves distinctly from its original form. These charged particles play a key role in chemical reactions, electrical conductivity, and the formation of various compounds. Understanding what defines these charged entities is essential to grasping how matter interacts at the atomic and molecular level.
An entity becomes charged when the number of electrons does not equal the number of protons. If there are more electrons, the particle becomes negatively charged, while fewer electrons result in a positive charge. This imbalance alters the behavior of the particle, affecting how it bonds with other substances and participates in different chemical processes. As such, these particles are integral to numerous phenomena in both chemistry and physics.
The ability of charged particles to interact with other species is crucial in various fields, from the operation of electrical circuits to the chemistry of living organisms. Their presence in solutions and reactions is vital for understanding the flow of energy and the formation of new substances. Thus, the concept of being a charged particle extends far beyond simple theory, influencing real-world applications across many domains.
How Atoms Become Ions
Atoms can transform into charged particles through the loss or gain of electrons. This change alters their overall electrical balance, turning them from neutral to positively or negatively charged. The process typically occurs when atoms interact with other substances, especially in reactions involving energy transfer. These alterations are key to understanding how matter behaves and interacts in various chemical processes.
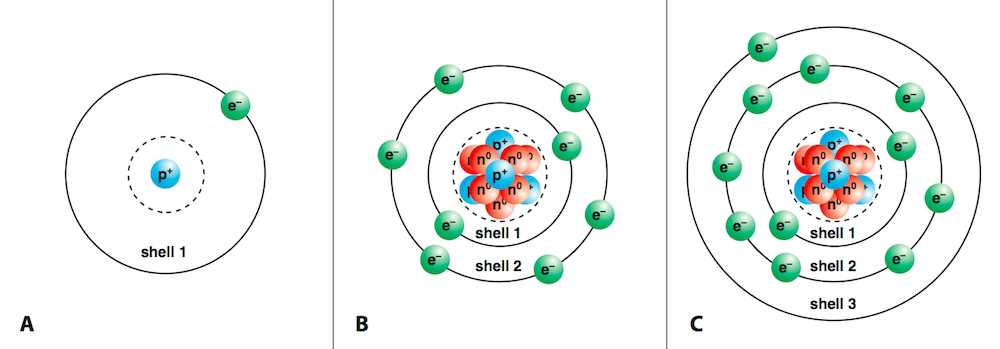
Atoms have a specific number of electrons surrounding their nucleus, usually balanced with the protons in the nucleus. However, under certain conditions, an atom may lose one or more electrons, resulting in a positive charge. Conversely, if an atom gains extra electrons, it becomes negatively charged. This imbalance in the number of electrons and protons is what distinguishes a charged particle from a neutral atom.
In many chemical reactions, such as those involving salts or acids, atoms undergo ionization. This transformation allows them to interact more readily with other particles, forming bonds and new compounds. The ability of atoms to change charge is central to many processes, including electrical conductivity, acid-base reactions, and even biological functions like nerve signaling.
The Role of Electrons in Ion Formation
Electrons play a critical part in the process that turns neutral atoms into charged particles. These tiny, negatively charged subatomic particles are involved in many interactions that determine an atom’s charge. When atoms gain or lose electrons, they change their overall electrical charge, becoming either positively or negatively charged. Understanding this role is key to grasping how elements behave in chemical reactions.
The Movement of Electrons
In an atom, electrons are arranged in energy levels or shells surrounding the nucleus. These electrons are loosely held in the outermost shell, especially in atoms with fewer electrons. When an atom undergoes a reaction or an external force is applied, electrons may be transferred to or from the atom, leading to a net charge. The more or fewer electrons an atom has compared to protons, the greater the imbalance, thus affecting the atom’s chemical properties and reactivity.
How Electron Loss or Gain Affects Charge
When an atom loses electrons, it becomes positively charged because it now has more protons than electrons. This process is known as oxidation. Conversely, if an atom gains electrons, it becomes negatively charged, a process referred to as reduction. The movement of electrons in these processes is crucial for the formation of compounds and the way atoms interact with each other in various chemical reactions.
Difference Between Cations and Anions
When atoms or molecules gain or lose electrons, they acquire an electrical charge. This results in two distinct types of charged particles: those with a positive charge and those with a negative charge. While both are charged, they differ in how they form and interact with other particles. Understanding these differences is essential for studying chemical reactions and the behavior of substances in various environments.
Cations: Positively Charged Particles
Cations are particles that have a positive charge, which occurs when an atom or molecule loses one or more electrons. This loss of negatively charged particles leads to an overall surplus of protons, giving the atom or molecule a positive charge. Cations are often attracted to negatively charged particles and play an important role in various chemical processes.
- Cations are typically metals, such as sodium (Na+) and calcium (Ca2+).
- They are often involved in forming ionic bonds with anions to create neutral compounds.
- Cations can influence properties like conductivity and solubility in solutions.
Anions: Negatively Charged Particles
Anions, in contrast, are particles that carry a negative charge. This negative charge results from an atom or molecule gaining one or more electrons. The addition of these electrons gives the atom or molecule an excess of negative charge, making it an anion. Anions play a crucial role in the formation of salts and the regulation of various chemical reactions in nature.
- Anions are often nonmetals, such as chloride (Cl–) and sulfate (SO42-).
- They are essential in neutralizing cations to form stable compounds like sodium chloride (NaCl).
- Anions are also involved in many biological processes, such as maintaining the body’s electrolyte balance.
In summary, cations and anions differ primarily in their charge. Cations have a positive charge due to electron loss, while anions have a negative charge due to electron gain. These differences are fundamental to the interactions between charged particles in chemical reactions and the formation of new compounds.
Why Ionization Happens in Chemistry

Ionization occurs in chemistry as a result of atoms or molecules interacting with energy, other substances, or changes in environmental conditions. This process is crucial because it leads to the formation of charged particles, which significantly influence chemical reactions. By gaining or losing electrons, atoms can alter their properties, allowing them to participate in a wide variety of processes, from conducting electricity to forming new compounds.
Energy and the Formation of Charged Particles
One of the main reasons ionization happens is the input of energy. When atoms absorb energy, such as heat or light, electrons in the atom may gain enough energy to overcome the attractive forces from the nucleus and leave the atom. This loss or gain of electrons creates charged particles. Energy can come from various sources, including thermal energy, electromagnetic radiation, or collisions with other particles.
Environmental Conditions and Reactions
In addition to energy, the surrounding environment plays a role in ionization. For example, in aqueous solutions, water molecules can interact with salts, causing them to dissolve and break apart into charged particles. The presence of certain substances or changes in temperature, pressure, or pH can also facilitate ionization. These changes can promote the dissociation of molecules into charged species, which then take part in chemical reactions that would not otherwise occur in their neutral state.
Methods for Expressing Ions in Reactions
In chemical reactions, the behavior of charged particles can be clearly represented to understand how substances interact. By showing the movement of electrons and the formation of charged species, chemists can predict and explain the outcome of various reactions. Several methods are commonly used to represent these particles, each providing a clearer picture of how atoms and molecules behave during transformation.
Common Representation Methods

There are several standard ways to depict charged particles during chemical reactions. Below is a summary of common methods used to represent charged entities and their interactions:
| Method | Description |
|---|---|
| Full Ionic Equation | This method shows all the particles involved in a reaction, including both charged and uncharged species, in their complete form. |
| Net Ionic Equation | In this representation, only the particles that participate directly in the reaction are shown, excluding spectator particles. |
| Electron Dot Diagrams | This method uses dots to represent electrons and shows how they are transferred or shared during a reaction. |
| Lewis Structures | These diagrams represent atoms and their bonding electrons, illustrating how charged particles form bonds in molecular reactions. |
These methods help chemists visualize and track how atoms and molecules interact, providing insights into the mechanisms behind chemical processes and the formation of new substances.
Identifying Ionic Compounds in Chemistry
Ionic compounds are formed when atoms transfer electrons to create charged particles that are held together by electrostatic forces. These compounds typically consist of a metal and a non-metal, and they have distinctive properties that can be used to identify them. Recognizing these characteristics is important for understanding the structure and behavior of materials in various chemical reactions.
Key Characteristics of Ionic Compounds
Ionic compounds possess several key features that differentiate them from other types of compounds. Some of the most noticeable traits include:
- High Melting and Boiling Points: Due to the strong electrostatic forces between the oppositely charged particles, ionic compounds tend to have high melting and boiling points.
- Electrical Conductivity: When dissolved in water or melted, ionic compounds can conduct electricity because their charged particles are free to move.
- Solubility in Water: Many ionic compounds are soluble in water, as the polar nature of water molecules helps separate the charged particles.
- Crystalline Structure: Ionic compounds often form regular, repeating patterns, resulting in solid crystals that are hard and brittle.
Methods for Identifying Ionic Compounds
Several methods can help determine whether a substance is an ionic compound. These include:
- Observation of Physical Properties: The high melting point and crystalline form can be a strong indication of an ionic compound.
- Conductivity Tests: Testing the conductivity of a substance in water or in molten form can reveal whether it contains charged particles capable of moving freely.
- Solubility Tests: Many ionic compounds dissolve easily in water, so testing for solubility can help identify them.
By understanding these properties and testing for them, chemists can confidently identify ionic compounds and explore their role in various chemical processes.
How Ionic Bonds Are Formed

Ionic bonds form through the transfer of electrons between atoms, resulting in the creation of charged particles. These bonds occur when one atom gives up one or more electrons to another atom, creating an attraction between the positively charged particles (cations) and the negatively charged particles (anions). This electrostatic attraction holds the atoms together in a stable structure, forming compounds with unique properties.
Electron Transfer and Charge Imbalance
The formation of ionic bonds begins with the transfer of electrons from one atom to another. Typically, metals with fewer electrons in their outer shell will lose electrons, while non-metals with more electrons in their outer shell will gain them. This results in a charge imbalance, where the metal atom becomes positively charged (cation) and the non-metal atom becomes negatively charged (anion). The resulting attraction between the oppositely charged particles forms the bond.
Formation of Stable Compounds
Once the bond is formed, the resulting ionic compound is stable due to the strong attraction between the cations and anions. This attraction holds the atoms together in a repeating lattice structure, which is characteristic of ionic compounds. The stability of the ionic bond ensures that the compound remains intact under various conditions, and its properties are dictated by the arrangement of charged particles within the structure.
Ionization Energy and Its Importance
The concept of energy required to remove an electron from an atom is a key factor in understanding chemical reactions and the behavior of elements. This energy influences how atoms interact with other atoms and how easily they can form various types of bonds. Higher ionization energy means that an atom is less likely to lose electrons, while lower energy makes electron removal easier. Understanding this concept helps in predicting an element’s reactivity and its role in different chemical processes.
Factors Affecting Ionization Energy
Several factors determine how much energy is needed to remove an electron from an atom:
- Atomic Size: As atoms get larger, the outermost electrons are further from the nucleus, making them easier to remove and lowering ionization energy.
- Nuclear Charge: Atoms with a higher number of protons have a stronger pull on their electrons, resulting in higher ionization energy.
- Electron Shielding: Inner electrons can “shield” outer electrons from the full attraction of the nucleus, making electron removal easier for larger atoms.
Why Ionization Energy Matters
Ionization energy plays a crucial role in determining the chemical properties of elements and their ability to form compounds. Some of its important implications include:
- Reactivity: Elements with low ionization energy tend to be more reactive, as they can easily lose electrons to form positive ions.
- Bond Formation: The energy required to remove electrons affects how elements bond with others, influencing the type of compounds they form.
- Periodic Trends: Ionization energy helps explain trends within the periodic table, such as why metals typically have lower ionization energy compared to non-metals.
By understanding ionization energy, scientists can predict how elements will behave in chemical reactions and what kinds of compounds they will form, making it a fundamental concept in chemistry.
How Ionic Equations Work in Reactions
Ionic equations are a simplified way of representing chemical reactions, focusing on the particles that directly participate in the reaction. They highlight the movement of charged particles, allowing chemists to better understand how substances interact at the molecular level. By omitting spectator ions–those that do not change during the reaction–ionic equations provide a clearer picture of the actual chemical changes that occur in the process.
In a typical reaction, substances dissolve in water, breaking apart into charged particles. These particles can then combine or react with other charged species to form new compounds. Ionic equations help by showing these interactions while ignoring the unreacted particles that do not affect the outcome of the reaction.
Steps to Write Ionic Equations
Writing ionic equations involves a few key steps:
- Write the balanced molecular equation: Begin by writing the full chemical equation, including all reactants and products.
- Separate the soluble compounds into ions: For compounds that dissolve in water, split them into their respective ions.
- Remove spectator ions: Identify ions that do not change during the reaction and remove them from the equation.
- Write the net ionic equation: What remains after removing spectator ions is the net ionic equation, showing only the particles involved in the chemical change.
Importance of Ionic Equations
Understanding how ionic equations work is crucial for grasping the behavior of substances in solution. These equations help chemists determine which ions are involved in forming products and how the different species interact with each other. They are particularly useful in studying precipitation reactions, acid-base reactions, and redox processes, where the transfer of charged particles plays a key role.
The Impact of Ionization on pH Levels
The process of ion formation plays a significant role in determining the acidity or alkalinity of a solution. As substances dissolve in water and dissociate into charged particles, the concentration of specific ions influences the overall pH of the solution. This relationship is central to many chemical processes, including biological reactions, industrial applications, and environmental systems. Understanding how the release of certain ions impacts pH can help predict how solutions behave and react under different conditions.
Effect of Hydrogen Ions on Acidity
The concentration of hydrogen ions (H+) in a solution directly affects its pH level. When an acid dissolves in water, it releases H+ ions, which increases the acidity of the solution. A higher concentration of hydrogen ions results in a lower pH, making the solution more acidic. Conversely, substances that produce fewer hydrogen ions or increase the concentration of hydroxide ions (OH–) tend to raise the pH, making the solution more basic or alkaline.
The Role of pH in Chemical Reactions
pH not only affects the properties of solutions but also influences the rate and outcome of many chemical reactions. In biological systems, for example, enzymes and proteins are highly sensitive to pH changes, which can affect their structure and function. Similarly, in industrial settings, maintaining the correct pH is crucial for optimal reactions in processes such as fermentation, water treatment, and chemical manufacturing. Ionization, through its influence on the concentration of H+ and OH– ions, is a key factor in controlling pH and, consequently, in regulating these important chemical processes.
Common Examples of Ion Formation
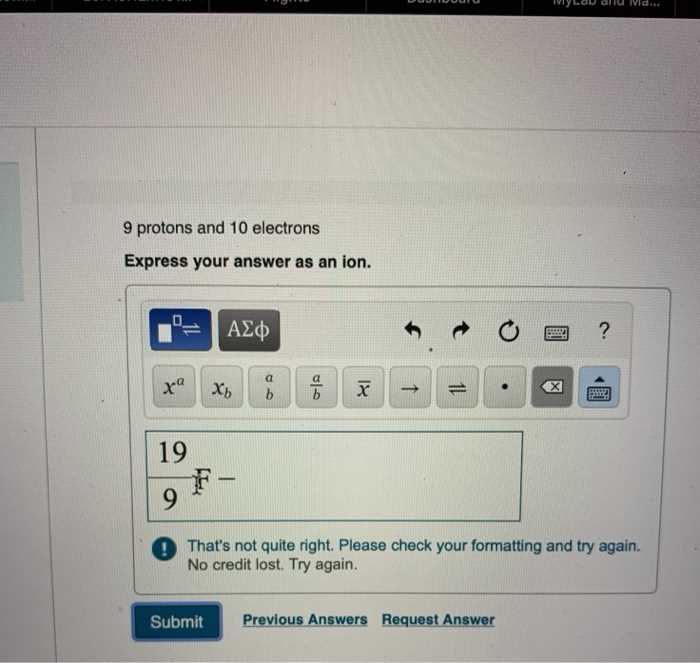
The formation of charged particles is a fundamental aspect of chemistry, occurring in various chemical processes and reactions. These particles, formed when atoms or molecules gain or lose electrons, are involved in many key reactions, from dissolving salts in water to complex biochemical processes. Below are some common examples where such transformations are observed, illustrating the diversity of reactions in which charged species play a central role.
Examples in Everyday Chemistry
Several everyday substances undergo transformations that result in the formation of charged particles. Some of the most well-known examples include:
| Substance | Reaction | Resulting Particles |
|---|---|---|
| Table Salt (NaCl) | Dissolves in water | Na+, Cl– |
| Hydrochloric Acid (HCl) | Dissolves in water | H+, Cl– |
| Calcium Chloride (CaCl2) | Dissolves in water | Ca2+, Cl– |
Biological Examples of Ion Formation
In biological systems, the formation and movement of charged particles is essential for numerous physiological functions, including nerve transmission and muscle contraction. Some common examples include:
| Biological Substance | Ion Movement | Function |
|---|---|---|
| Sodium (Na+) | Moves into cells | Facilitates nerve impulses |
| Potassium (K+) | Moves out of cells | Regulates heartbeats and muscle contractions |
| Calcium (Ca2+) | Moves into cells | Triggers muscle contractions |
These examples highlight the importance of charged particles in both everyday chemical processes and complex biological systems. The movement and interaction of these particles are central to understanding many natural and industrial phenomena.
Applications of Ions in Everyday Life
The movement and transformation of charged particles play a crucial role in numerous aspects of daily life. These particles, formed when atoms or molecules lose or gain electrons, are involved in everything from household products to biological processes. Understanding how these charged species work allows us to appreciate their importance in various technologies, health-related applications, and even environmental systems.
One of the most visible uses of these particles can be found in the batteries that power everyday devices. In batteries, the flow of charged particles between electrodes generates electricity. Similarly, the process of water purification relies on the ability of charged substances to attract and remove impurities. Many cleaning agents also make use of charged molecules to break down stains or kill bacteria.
In medicine, charged species are essential for treatments and diagnostic tools. For example, in treatments like dialysis, charged particles are used to remove waste from the body. Similarly, medical imaging techniques such as X-rays or MRI scans depend on the interaction of charged particles with body tissues to create detailed images.
In nature, the movement of charged particles is responsible for processes such as plant growth, nerve function, and muscle contraction. Whether it’s the transmission of electrical signals across neurons or the absorption of nutrients in plants, these particles are at the core of biological systems that sustain life.
These examples illustrate just a few of the many ways in which charged particles impact our world. From powering devices to maintaining biological functions, their presence is essential to both technological advancements and natural processes.
Ionization in Acid-Base Reactions

In chemical reactions involving acids and bases, the transformation of certain molecules into charged species plays a key role in the reaction’s dynamics. During these processes, compounds often break apart or dissociate into charged particles, influencing the overall behavior of the solution. This behavior is fundamental to understanding the properties of acids, bases, and their interactions in various environments, from laboratory settings to biological systems.
When an acid dissolves in water, it releases positively charged particles, which can significantly affect the solution’s characteristics. Similarly, bases tend to generate negatively charged particles, altering the pH balance. The interaction between these charged species–whether through attraction or repulsion–determines whether a reaction will proceed and what products will be formed.
The Role of Acids
Acids, when mixed with water, undergo a process in which they lose certain components, leading to the formation of positively charged particles. This increase in positive charge results in a more acidic solution, which has significant implications for reaction rates, solubility, and chemical stability.
The Role of Bases
Bases, on the other hand, act by gaining components from water, releasing negatively charged particles. The presence of these negatively charged species raises the pH of the solution, making it more alkaline. The ability of bases to neutralize acids and vice versa is a result of the interaction between these charged species, driving the overall chemistry of acid-base reactions.
These processes of transformation and dissociation are crucial for a wide range of chemical reactions, including those that take place in living organisms, industrial applications, and even household products. The understanding of how acids and bases interact through the release of charged particles helps explain many everyday phenomena, such as the buffering capacity of blood or the cleaning properties of soaps and detergents.
Understanding the Charge of Ions
The charge of charged particles, formed through the loss or gain of electrons, plays a crucial role in their behavior in chemical reactions and interactions. These charged species, whether positively or negatively charged, interact with each other based on their charge, influencing everything from molecular structure to physical properties. Understanding the nature of these charges is essential in fields ranging from chemistry and biology to environmental science.
When atoms or molecules gain electrons, they acquire a negative charge, while the loss of electrons results in a positive charge. This ability to gain or lose electrons is not only fundamental to the formation of charged species, but also governs the strength of their interactions with other particles, including solvents, other charged species, and even solid materials.
Positive Charge: Cations
Particles that lose electrons are referred to as positively charged species. These positively charged particles, known as cations, tend to be attracted to negatively charged particles in solutions. The magnitude of the positive charge depends on how many electrons the atom or molecule has lost. For example, a sodium atom (Na) can lose one electron to form Na+, whereas calcium (Ca) can lose two electrons to form Ca2+.
Negative Charge: Anions
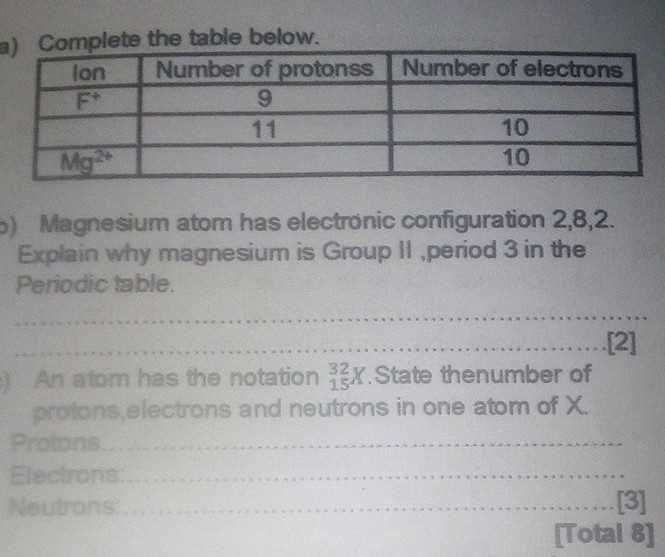
On the other hand, when particles gain electrons, they become negatively charged, known as anions. These particles are attracted to positively charged entities, and the charge is determined by the number of electrons gained. For instance, when a chlorine atom (Cl) gains one electron, it forms a chloride anion (Cl–). Understanding the distribution and balance of positive and negative charges is essential in many chemical processes, such as in the formation of salts and in maintaining biological functions like nerve transmission and muscle contraction.
The behavior of charged species in different environments, including how they interact with other substances, is largely dictated by their charge. This understanding provides insight into the chemical processes that shape everything from the conductivity of solutions to the formation of complex biological molecules.
How to Express Ions in Chemical Notation
In chemical reactions and formulas, representing charged particles is essential for understanding their behavior and interactions. These particles can either carry a positive or negative charge, and their notation follows specific conventions to clearly indicate their nature. Knowing how to properly represent these charged species is vital for balancing chemical equations and interpreting reaction mechanisms.
Typically, the notation for charged species includes the symbol of the element or compound, followed by its charge. The charge is written as a superscript number and sign: a positive charge is indicated with a “+” sign, while a negative charge is represented with a “-” sign. The number of charges is also denoted as a superscript, if necessary, to show the magnitude of the charge.
Representing Positive Charged Species (Cations)
Cations are particles that have lost electrons and therefore carry a positive charge. When denoting a cation in chemical notation, the following format is used:
- The element’s symbol is written first.
- The charge is indicated as a superscript with a “+” sign. If the particle has a charge greater than 1, the magnitude of the charge is also included.
For example:
- Na+ (Sodium ion) – indicates a single positive charge.
- Ca2+ (Calcium ion) – indicates a double positive charge.
Representing Negatively Charged Species (Anions)
Anions are particles that have gained electrons, resulting in a negative charge. The notation for anions follows a similar structure:
- The element’s symbol is written first.
- The charge is shown as a superscript with a “-” sign. The magnitude of the negative charge is included if necessary.
For example:
- Cl– (Chloride ion) – indicates a single negative charge.
- SO42- (Sulfate ion) – indicates a double negative charge.
This method of notation allows chemists to quickly identify the charge and type of each particle involved in a reaction, making it easier to balance equations and predict reaction outcomes.
Real-World Examples of Ionization
Ionization is a fundamental process that occurs in various natural and industrial phenomena. Understanding how certain materials lose or gain electrons can provide insights into many aspects of science, technology, and everyday life. The process of forming charged particles is central to a wide range of chemical reactions, environmental processes, and technological applications.
One of the most common examples of ionization can be observed in the dissociation of salts in water. When a salt like sodium chloride (NaCl) is dissolved in water, it separates into sodium ions (Na+) and chloride ions (Cl–). This dissociation is crucial for many biological processes, such as nerve signal transmission and maintaining proper hydration levels in cells.
Another well-known example occurs in the process of acid-base reactions. When acids like hydrochloric acid (HCl) dissolve in water, they ionize to form hydrogen ions (H+) and chloride ions (Cl–). These hydrogen ions are responsible for the acidic properties of the solution, which play a critical role in digestion and various industrial applications, such as cleaning and manufacturing.
In the field of chemistry, ionization also occurs during the interaction of gases with electric fields. For instance, when a gas like oxygen (O2) is exposed to high-energy photons or electric fields, it can lose electrons and become ionized, forming charged particles like O2+. This process is a key component of plasma technology, which has applications in everything from neon signs to advanced industrial processes like semiconductor fabrication.
Ionization is also critical in everyday technologies, such as batteries and fuel cells. In a typical battery, an electrochemical reaction occurs that involves the movement of charged particles between two electrodes, resulting in the generation of electric current. This movement of ions is essential for the operation of many portable devices, from mobile phones to electric vehicles.
These examples highlight how ionization is integral to both natural processes and modern technology. By gaining or losing electrons, particles become more reactive and capable of participating in the diverse chemical reactions that drive life and industry.