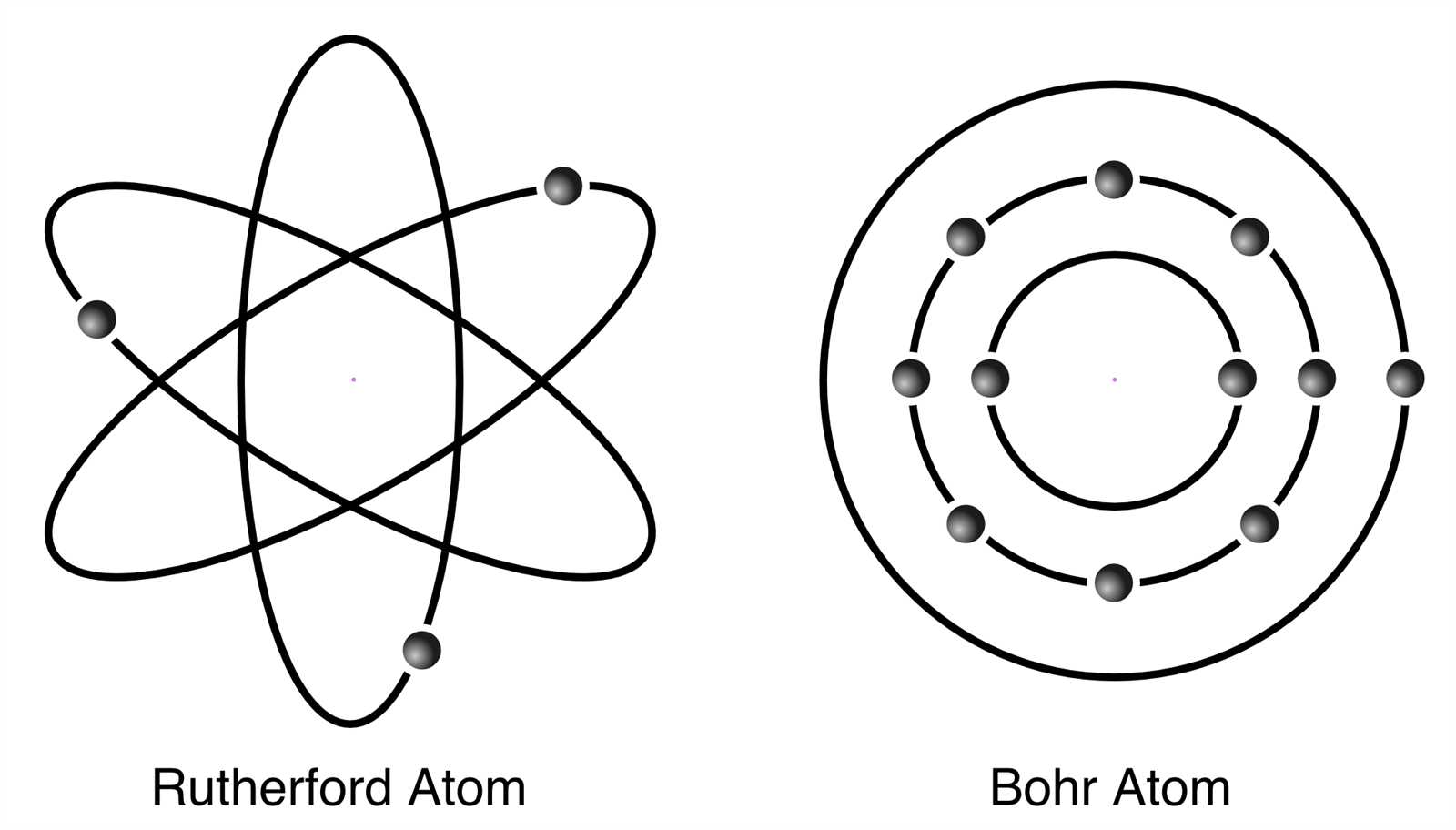
The organization of particles within a substance plays a crucial role in determining its properties and behavior. By examining how these particles are positioned and interact with one another, scientists can unlock the mysteries of chemical reactions, material strengths, and more. This section explores how the fundamental building blocks are distributed, providing insight into the forces that govern their arrangement.
At the core of this exploration lies the concept of energy levels and sublevels, which define how these particles are grouped and how they contribute to the overall structure of a substance. The principles guiding their placement not only shape the behavior of matter but also influence the formation of bonds between different substances. A deeper understanding of this organization allows us to predict chemical behaviors and apply this knowledge in practical fields like engineering, medicine, and technology.
Understanding Electron Configuration Basics
The way particles within a substance are organized plays a vital role in its overall structure and behavior. These tiny components are grouped in specific patterns that determine how a material reacts, bonds with others, and behaves under different conditions. This concept lies at the heart of understanding matter on a deeper level, as it influences a variety of properties from conductivity to reactivity.
Each particle exists within certain energy levels, and these levels are further divided into regions that hold a set number of these components. These regions or sublevels define how particles are distributed, and the way they occupy these spaces helps shape the characteristics of the material. The way this distribution follows specific rules is fundamental to the structure of all substances, allowing scientists to predict how they will interact with one another.
Recognizing these patterns and principles not only helps explain the behavior of different elements but also allows for the application of this knowledge in real-world situations. Whether in chemical reactions, material science, or energy production, understanding how components are organized is essential for numerous scientific and practical fields.
Atomic Structure and Electron Shells
The internal composition of a substance is built on a core structure, where the smallest units are arranged in a specific layout. These units are surrounded by layers that help define the behavior of the material. The arrangement of these layers influences the way the substance reacts with others and how it maintains its stability. Understanding this structure is essential for explaining many chemical and physical properties.
Energy Levels and Their Significance
The central component, often referred to as the nucleus, is surrounded by multiple layers that are filled with distinct groups of particles. These layers are organized in a way that each holds a particular number of these particles. The outermost of these layers plays a key role in how the substance interacts with other materials, as they determine its ability to bond and form connections with surrounding elements.
Sublevels and Their Distribution
Each layer consists of subregions, where these components are more tightly grouped. These sublevels are not all equivalent; each one has a different capacity for holding these particles. Their distribution follows precise rules that define the overall stability of the structure, impacting the substance’s chemical behavior and physical properties.
The Role of Electrons in Atoms
The smallest components that form a substance play a crucial role in its overall behavior and characteristics. These components are responsible for a variety of interactions that influence how materials react with one another, bond, and undergo changes. Understanding the specific functions of these components is vital for explaining many physical and chemical phenomena.
Key Functions of the Components
These components, located in various layers surrounding the nucleus, serve several important purposes:
- Bond Formation: They are essential in the creation of bonds between different substances, helping form compounds and molecules.
- Electrical Conductivity: The movement and arrangement of these particles in certain layers determine a material’s ability to conduct electricity.
- Reactivity: Their interaction with components from other substances dictates how a material will react in chemical reactions.
Impact on Chemical Behavior
The outermost layers, where these components are often found, are especially important in defining how substances behave. These layers are the most involved in chemical interactions, affecting everything from how materials combine to the stability of the resulting compounds. Their distribution, or how they are spread across different regions, determines the unique properties of each substance.
Electron Energy Levels and Orbitals
The internal structure of a substance is organized into distinct regions where its smallest components are located. These regions, often referred to as energy levels, dictate the overall stability and behavior of the material. The components within each level are not randomly distributed, but rather follow specific patterns that influence how they interact and bond with other substances.
Each energy level is capable of holding a certain number of these components, with higher levels having a greater capacity. Within these levels, the components are further organized into subregions, known as orbitals, where they are most likely to be found. These orbitals play a critical role in determining the material’s chemical properties, as the arrangement of these regions affects how substances interact with one another and form bonds.
Quantum Mechanics and Electron Behavior
The behavior of the smallest components within a substance is governed by principles of quantum mechanics, which offer a framework for understanding their movement and interaction. Unlike classical physics, quantum mechanics describes how these particles exist in multiple states at once, only “choosing” a specific state when measured or observed. This idea introduces a level of unpredictability and complexity in how these components behave, especially at the microscopic level.
Quantum theory also explains how these components occupy specific regions within a substance, with each region being associated with distinct energy levels. These particles do not move in fixed paths; instead, they exist in a cloud-like probability distribution, which describes where they are most likely to be found. This behavior, governed by wave functions and energy states, plays a crucial role in understanding chemical reactions, material properties, and the formation of bonds between substances.
Building Blocks of Electron Arrangements
The fundamental components that form the structure of any substance are organized in specific ways that influence how they interact and behave. These smallest units, which make up all matter, follow a set of rules governing their distribution across different regions or levels. Understanding how these components fit together within these levels helps explain a variety of chemical and physical behaviors.
The key building blocks are the energy levels and sublevels that define where these components are most likely to be found. These regions are not uniform but consist of different types of spaces, each capable of holding a specific number of components. The distribution across these spaces is critical for determining how a substance reacts and forms bonds with others.
| Energy Level | Sublevel | Capacity |
|---|---|---|
| 1 | s | 2 |
| 2 | p | 6 |
| 3 | d | 10 |
| 4 | f | 14 |
Energy Sublevels and Their Impact
The way components are distributed within different energy regions significantly influences the behavior of substances. These regions are divided into sublevels, each capable of holding a specific number of these components. The arrangement within these sublevels affects how the substance interacts with others and dictates many of its chemical and physical properties.
Each sublevel plays a distinct role in determining the material’s stability, reactivity, and ability to bond with other substances. These subregions are organized based on their energy capacity and proximity to the core. The specific filling order of these sublevels contributes to the formation of bonds and the material’s overall behavior under various conditions.
| Energy Level | Sublevel | Capacity |
|---|---|---|
| 1 | s | 2 |
| 2 | p | 6 |
| 3 | d | 10 |
| 4 | f | 14 |
Pauli Exclusion Principle in Action
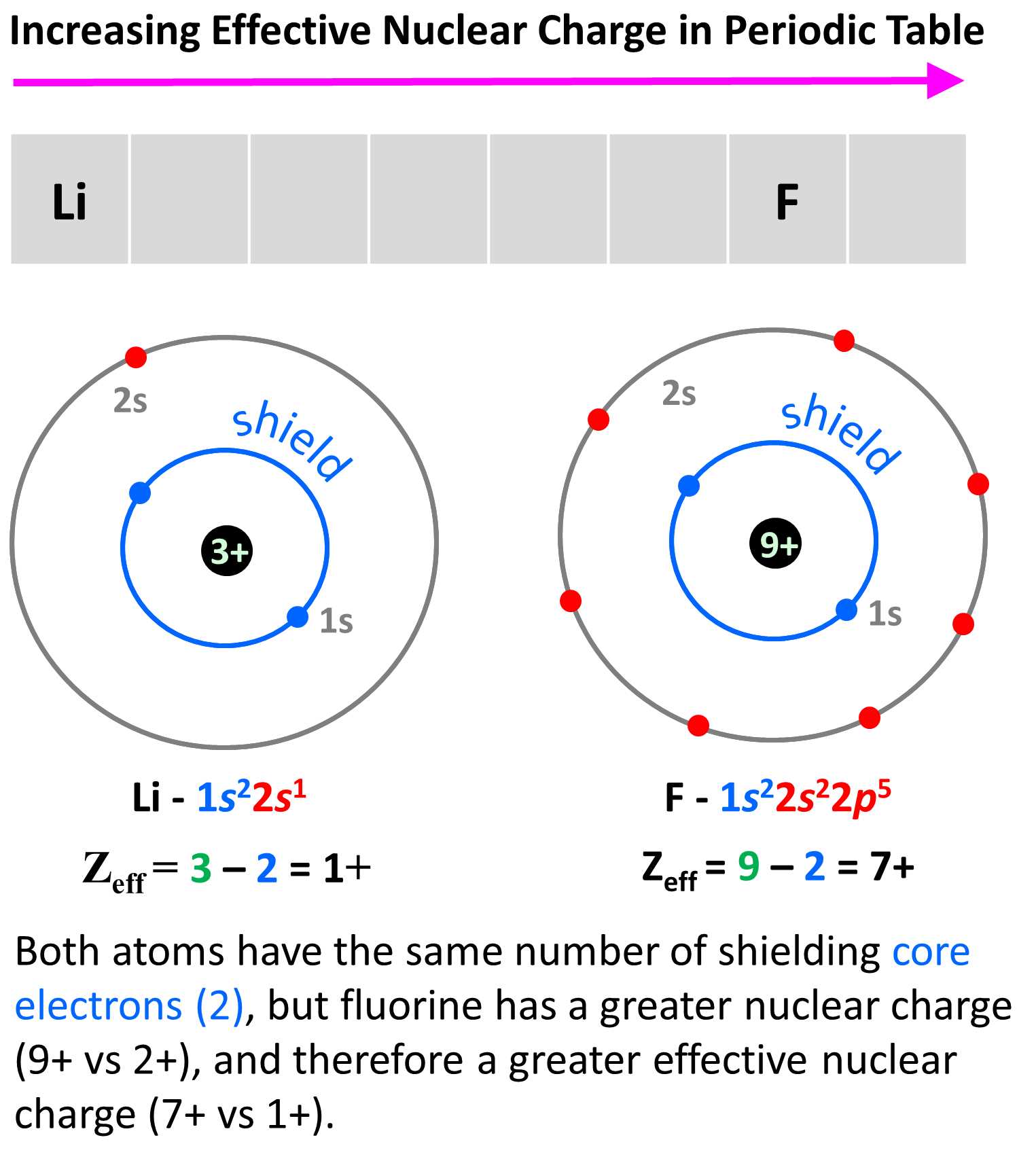
The fundamental behavior of components within a substance is governed by a set of rules that ensure no two of these particles share identical characteristics in the same space. This concept, known as the Pauli Exclusion Principle, plays a key role in determining how these particles fill available regions and subregions. It helps explain the stability and structure of matter at a microscopic level.
According to this principle, each particle must occupy a unique state, meaning that no two can share the same combination of properties, such as energy and spatial orientation, within a given region. This restriction forces the particles to spread out, occupying different sublevels and influencing how they interact with one another. As a result, the principle is crucial in shaping the chemical and physical behavior of materials.
Key consequences of this principle:
- Stability of Matter: Ensures that particles fill available spaces efficiently, preventing overcrowding in any given region.
- Chemical Properties: Impacts how substances bond and react based on the unique positions of these particles.
- Energy Distribution: Leads to a specific distribution pattern of particles across different energy levels and sublevels.
Hund’s Rule and Electron Distribution
The distribution of the smallest components within a material follows specific patterns that help define its behavior. One of the key principles governing this distribution is Hund’s Rule, which ensures that particles are arranged in a way that maximizes stability. This rule states that when multiple subregions are available, these components will first occupy separate spaces, rather than pairing up, to minimize energy and enhance stability.
By applying this rule, the system ensures that the particles are as spread out as possible within available subregions. This distribution leads to a more stable configuration, as the system attempts to lower its overall energy. Understanding how Hund’s Rule affects the arrangement of particles helps explain the structure of substances and their reactivity in chemical reactions.
Key points of Hund’s Rule:
- Maximization of Unpaired Components: Components will occupy different subregions before pairing up, ensuring a more stable configuration.
- Minimization of Energy: The rule helps distribute components in a way that reduces the system’s total energy.
- Influence on Chemical Properties: This distribution affects how substances interact with one another and form bonds.
Electron Configuration Notation Explained
In order to describe the distribution of the smallest components within a substance, a shorthand system is often used. This system allows for the quick representation of how these particles are arranged in various regions or sublevels. The notation is based on specific rules that reflect the order in which particles fill these spaces, making it easier to predict and understand a substance’s behavior.
This system of notation utilizes numbers, letters, and superscripts to indicate the energy levels, sublevels, and the number of particles in each sublevel. By following a set pattern, the notation allows scientists and chemists to easily communicate complex information about the internal structure of materials.
Key features of the notation:
- Energy Levels: Represented by numbers (e.g., 1, 2, 3) that correspond to specific regions within a substance.
- Sublevels: Indicated by letters (s, p, d, f) that denote the type of space within an energy level.
- Superscripts: Used to show the number of components present in a particular sublevel.
For example, a typical notation like “2p6” would indicate that the second energy level has the p sublevel, and there are six particles in that subregion.
Electron Configuration for Different Elements
Each substance is made up of a unique combination of components that are distributed across different energy levels and subregions. The configuration of these components varies from one element to another, and understanding how they are arranged can help explain the chemical and physical properties of each element. By looking at the configuration, one can predict an element’s reactivity, bonding behavior, and other important characteristics.
For each element, the distribution follows a specific order based on the increasing energy of the available spaces. The way in which particles fill these spaces influences the overall behavior of the element, such as its ability to form bonds, its reactivity with other substances, and its position in the periodic table.
Example of the First Few Elements
For lighter elements like hydrogen and helium, the particles occupy the first energy level. As elements increase in size, the configuration becomes more complex, with particles filling higher energy levels and various sublevels. For instance, hydrogen has one particle in the first level, while helium has two particles in the same level, filling it completely.
Configuration for Heavier Elements
As we move to heavier elements, the distribution becomes more intricate. Elements like carbon and oxygen require multiple energy levels, with particles filling the sublevels according to specific rules. The way these particles fill sublevels determines the chemical properties, such as electronegativity and ionization energy, which vary widely among elements.
Common Patterns in Electron Arrangements
The distribution of the smallest components in different substances often follows recognizable patterns that are influenced by various principles. These patterns help explain the behavior of elements and provide insight into their chemical properties. By understanding these regularities, scientists can predict how elements will interact, bond, and react with one another.
Over time, researchers have identified several trends in the way these particles fill available regions. These trends are particularly evident in the periodic table, where elements with similar configurations tend to exhibit similar properties. For instance, elements in the same group of the periodic table often show similar reactivity and bonding behavior due to their similar internal structure.
Trends in the Periodic Table
Elements in the same column of the periodic table tend to have similar distributions of their smallest components, resulting in similar chemical properties. This is because they have the same number of components in their outermost regions. For example, alkali metals all have a single component in their outermost sublevel, making them highly reactive and prone to bonding with other substances.
Filling Order of Energy Levels
As elements increase in size, the filling of their energy levels follows a set order, starting from the lowest energy level and moving outward. This process is governed by the principles of quantum mechanics, ensuring that lower energy levels are filled before higher ones. The table below illustrates how the distribution follows a predictable order as elements grow in complexity.
| Element | Configuration | Notable Feature |
|---|---|---|
| Hydrogen | 1s1 | First energy level, single component |
| Helium | 1s2 | Full first energy level |
| Lithium | 1s22s1 | Begins filling the second energy level |
| Carbon | 1s22s22p2 | Second energy level with 2s and 2p sublevels filled |
Electron Configuration and Chemical Properties
The way in which subatomic particles are distributed across different energy levels plays a crucial role in determining the characteristics of a substance. This internal structure influences how an element interacts with others, its tendency to form bonds, and its reactivity. By examining the configuration of these components, scientists can predict a wide range of chemical behaviors, including how an element might react in a chemical reaction or what types of compounds it is likely to form.
As elements have unique configurations of their components, this directly impacts their position in the periodic table and their chemical behavior. For example, elements with similar distributions in their outer regions tend to share similar properties. This is why elements in the same group, such as the halogens or noble gases, display comparable reactivity and bonding tendencies.
The number of components in an element’s outermost region, or its “valence” particles, is one of the most significant factors that determines how it will behave chemically. Elements with similar valence configurations tend to behave similarly. This is why elements in the same group of the periodic table often have similar chemical reactivity. For example, alkali metals, which all have a single particle in their outermost sublevel, are highly reactive and readily form bonds with other substances.
Exceptions to the Electron Configuration Rules

While the general principles that govern the distribution of particles across various energy levels are well-established, there are certain instances where these rules do not apply as expected. These exceptions arise due to the complex interactions between the subatomic components, particularly when energy minimization leads to alternative configurations. As a result, some elements exhibit unexpected behaviors that deviate from the typical filling order of energy levels and sublevels.
One notable example occurs in transition metals, where the configuration may not follow the expected pattern due to the stability associated with partially filled sublevels. This phenomenon often results in electrons being distributed in a way that minimizes the overall energy of the system, leading to more stable configurations. Similarly, some elements in the lanthanide and actinide series also show irregularities due to their unique electron interactions and the peculiarities of their inner electron shells.
These deviations can affect the chemical properties of elements, influencing their reactivity, magnetic behavior, and bonding capabilities. Understanding these exceptions is crucial for chemists when predicting the behavior of elements in various chemical reactions or in the formation of complex compounds.
Importance of Electron Configurations in Bonding
The way subatomic particles are distributed across different regions plays a vital role in how elements interact with each other, especially when it comes to forming bonds. This distribution governs how strongly elements are attracted to others, which in turn influences the types of bonds they form and the overall stability of molecules. Understanding the distribution pattern of these particles is key to predicting how substances will behave in chemical reactions.
When particles in the outermost regions of an element are involved in bonding, the distribution of these particles largely determines whether an element will form ionic, covalent, or metallic bonds. For example:
- Ionic bonds: Elements with few particles in their outer shell tend to lose them and form positive ions, while elements with nearly full outer shells gain electrons to form negative ions. These oppositely charged ions are attracted to each other, forming a strong bond.
- Covalent bonds: When two elements have similar tendencies to attract particles, they may share particles to achieve stability, forming a covalent bond. This occurs when both elements prefer to “hold on” to their particles rather than giving them away.
- Metallic bonds: In metallic bonding, elements with loosely held particles form a “sea” of delocalized particles that move freely, contributing to the electrical conductivity and malleability of metals.
In conclusion, the arrangement of subatomic components in an element determines how it interacts with others, directly influencing its ability to form stable chemical bonds. By understanding these patterns, scientists can better predict the properties of compounds and their behavior in chemical reactions.
Visualizing Electron Configurations in Diagrams

Understanding the distribution of subatomic particles within different regions of an element is crucial to comprehending its chemical behavior. Diagrams offer an intuitive way to visualize how these components are arranged, making complex concepts easier to grasp. Through various types of diagrams, such as energy level diagrams and orbital notation, it becomes clearer how particles are positioned in different shells and orbitals.
One common way to represent this distribution is through orbital diagrams, where each orbital is depicted with arrows representing particles. This method highlights not only the number of particles in each orbital but also their relative orientations. Below is a basic example of how energy levels and orbitals might be displayed:
| Energy Level | Orbital Type | Diagram Representation |
|---|---|---|
| 1 | s | ↑↓ |
| 2 | p | ↑↓ ↑↓ ↑ |
| 3 | d | ↑↓ ↑↓ ↑↓ ↑ ↑ |
| 4 | f | ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ |
In this table, the arrows (↑ and ↓) represent the presence of particles in orbitals, with the direction indicating their spin. This method helps to visually track the arrangement of components and provides a clearer understanding of how particles fill available spaces in shells and orbitals.
These diagrams are essential tools in chemistry, aiding students and researchers in predicting the behavior of elements in various chemical processes. By visualizing configurations, it becomes easier to analyze bonding tendencies, reactivity, and the formation of compounds.
Practical Applications of Electron Arrangement
The distribution of subatomic particles within different regions of a substance plays a critical role in determining its chemical and physical properties. Understanding how these particles are positioned helps scientists and engineers design materials with specific characteristics and predict how substances will behave in various environments. In this section, we explore some of the real-world applications of this knowledge and its impact on a wide range of fields.
Material Science and Engineering
By understanding how components fill energy levels and orbitals, material scientists can develop substances with tailored properties. For instance, the conductivity, strength, and reactivity of metals and nonmetals are influenced by their subatomic composition. Here are a few examples:
- Semiconductor Technology: Knowledge of particle distributions allows for the creation of semiconductor materials used in microelectronics.
- Superconductivity: Specific configurations of particles enable certain materials to exhibit superconductivity at low temperatures.
- Alloy Design: Adjusting the subatomic makeup of alloys leads to the creation of stronger, more durable materials for use in various industries.
Chemistry and Chemical Reactions
In chemistry, the way particles are distributed influences how substances interact during chemical reactions. By understanding these distributions, scientists can predict the reactivity of elements, design new chemical compounds, and even develop catalysts for industrial processes. Some key applications include:
- Pharmaceuticals: Drug molecules are designed to interact with specific sites in biological systems, a process influenced by the distribution of subatomic particles.
- Catalysis: By manipulating the particle configuration in catalysts, reactions can be sped up or made more efficient in industrial settings.
- Environmental Chemistry: Understanding how particles interact with pollutants can help in designing more effective filtration and remediation technologies.
In summary, knowledge of how subatomic components are arranged not only drives advances in technology but also enables a deeper understanding of the natural world. It empowers professionals across many disciplines to solve complex problems and create innovations that improve everyday life.