
In the study of life sciences, the movement of substances across cell membranes plays a critical role in maintaining balance within living organisms. These processes ensure that essential nutrients and gases enter cells, while waste products exit, enabling proper function.
Understanding how materials move between different environments is essential for solving various scientific inquiries. These mechanisms occur naturally, influenced by factors such as concentration differences and temperature. Mastery of these concepts is vital for tackling challenges in biology, particularly in understanding cellular behavior and environmental interactions.
By examining real-world examples, one can gain insight into how the principles of movement apply across different biological systems. Whether it’s the transport of gases in lungs or water absorption in roots, these mechanisms are fundamental to life itself. Recognizing the underlying principles helps to clarify common questions faced by students and researchers alike.
Understanding Diffusion and Osmosis
These essential processes govern how substances move within living organisms. Whether it’s the transfer of gases or the movement of water, understanding the mechanisms that drive these actions is crucial for explaining many biological functions. Both rely on the movement of molecules, but they occur through different methods, each with its unique characteristics.
One process involves the natural tendency of molecules to spread out from areas of high concentration to those with low concentration. This is a key principle that ensures cells receive the nutrients they need while removing waste efficiently. The second mechanism specifically focuses on the movement of water across a membrane, driven by concentration differences of solutes on either side.
Both mechanisms are integral to cellular life, playing a significant role in maintaining equilibrium within organisms. The movement of molecules through various environments impacts everything from nutrient absorption in plants to the regulation of water balance in animals.
What is Diffusion in Biology
This natural phenomenon plays a fundamental role in the movement of molecules within living systems. It is responsible for ensuring that cells obtain essential substances from their surroundings while simultaneously eliminating waste. Without this process, cells would not be able to maintain the necessary balance for survival and growth.
Key Characteristics of Molecular Movement
The process involves the spontaneous movement of particles from areas with a high concentration to those with a low concentration. This occurs until equilibrium is reached, where the molecules are evenly distributed across the available space. The driving force behind this movement is the inherent kinetic energy of the particles.
Importance in Living Organisms
In biological systems, this mechanism is essential for numerous functions, such as gas exchange in the lungs, nutrient uptake in cells, and the elimination of metabolic waste. Without effective molecular movement, organisms would be unable to perform the vital processes that support life.
Principles of Osmosis Explained
This vital process involves the movement of water molecules across a selectively permeable barrier. The movement occurs in response to differences in solute concentration, helping to maintain balance within biological systems. It is essential for regulating fluid levels and ensuring that cells function properly in various environments.
Selective Permeability of Membranes
The process relies on the ability of membranes to allow only specific molecules to pass through while blocking others. Water molecules are small enough to move freely through these barriers, but larger solute particles are restricted. This selective permeability is key to the mechanism that ensures water moves toward areas with higher solute concentrations.
Impact on Cellular Function
For living organisms, the movement of water helps maintain internal pressure within cells and tissues. It ensures that cells can absorb nutrients, expel waste, and maintain their shape. The movement of water is critical for many physiological processes, such as nutrient transport in plants and the regulation of blood volume in animals.
Key Differences Between Diffusion and Osmosis
While both processes involve the movement of particles from areas of higher to lower concentration, they differ in several important ways. These mechanisms are essential for maintaining homeostasis in living organisms, but their underlying principles and the types of particles they involve set them apart.
Process Involvement
- Movement of molecules: The first mechanism typically involves the movement of all types of molecules, including gases, liquids, and solids, across a membrane or barrier.
- Water movement: The second process specifically focuses on the movement of water molecules across a semi-permeable membrane.
Key Factors in Both Mechanisms
- Concentration gradient: Both mechanisms are driven by differences in concentration, but one involves solutes, while the other is concerned with solvent (water) movement.
- Energy requirement: Neither process requires external energy input, relying on the natural kinetic energy of molecules for movement.
Real-Life Examples of Diffusion
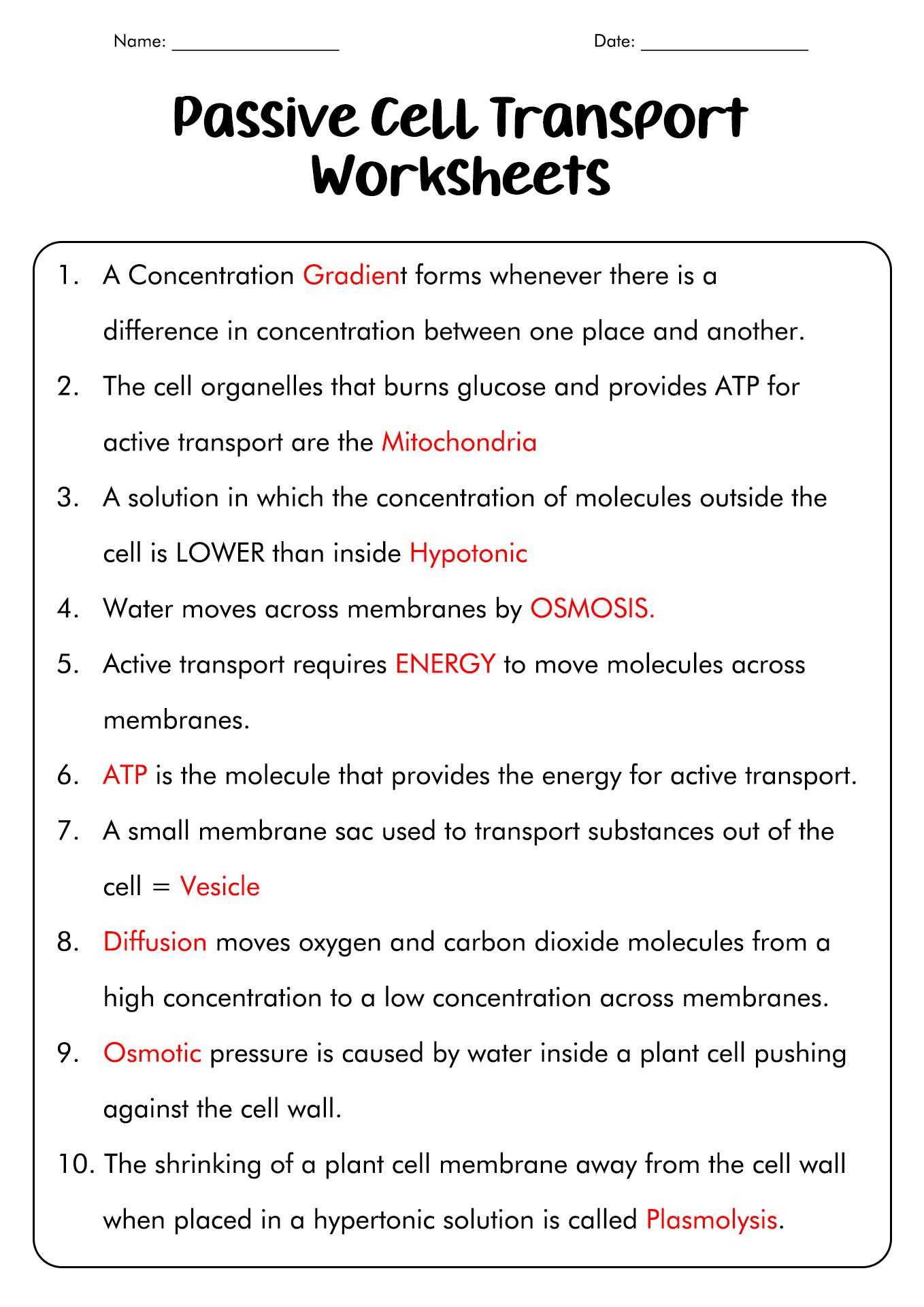
The movement of particles from areas of higher to lower concentration occurs naturally in various everyday situations. This process plays a crucial role in numerous biological and environmental phenomena, ensuring the proper functioning of systems in both living organisms and the surrounding environment.
Here are some examples where this mechanism is evident in the natural world:
| Example | Explanation |
|---|---|
| Perfume in the Air | When a bottle of perfume is opened, its scent molecules spread out, gradually filling the room. This is an example of particles moving from high to low concentration in the air. |
| Gas Exchange in Lungs | In the lungs, oxygen moves from areas of higher concentration in the air sacs to lower concentration in the blood, while carbon dioxide moves in the opposite direction. |
| Food Coloring in Water | When a drop of food coloring is added to water, the color gradually spreads throughout the liquid, as molecules move from a concentrated spot to fill the entire container. |
| Salt in Water | When salt is added to water, its particles spread out to dissolve evenly, moving from a concentrated area to disperse throughout the liquid. |
How Osmosis Affects Plant Cells
The movement of water molecules across a semi-permeable membrane has a significant impact on the structure and function of plant cells. This process ensures that cells maintain the proper amount of water, which is vital for their survival and growth. Without it, plants would be unable to transport nutrients or maintain the necessary internal pressure within their cells.
Maintaining Turgor Pressure
In plant cells, the movement of water into the cell causes the cell to swell, creating pressure against the cell wall. This pressure, known as turgor pressure, helps maintain the cell’s rigidity and structural integrity. Turgor pressure is essential for keeping plants upright and allows them to stand tall even without a skeleton.
Effect of Water Loss on Plant Cells
If a plant loses too much water, the cells lose turgor pressure, causing them to shrink. This can lead to wilting, a common sign of dehydration in plants. Without sufficient water intake, plants may struggle to take in nutrients, which can affect their growth and overall health.
Factors Influencing Diffusion Rates
The rate at which molecules move from an area of high concentration to an area of low concentration depends on several factors. These variables play a crucial role in determining how quickly substances spread within different environments, affecting everything from biological processes to chemical reactions.
Temperature
Higher temperatures increase the energy of molecules, causing them to move faster. This leads to an increase in the rate at which particles spread out. In living systems, this is particularly important for processes like nutrient uptake and waste removal, which require efficient movement of molecules.
Concentration Gradient

The larger the difference in concentration between two areas, the faster particles will move. When there is a steep gradient, molecules are driven to move more rapidly in an attempt to reach equilibrium. This factor is key to many physiological processes, such as the exchange of gases in the lungs or the transport of substances into cells.
Understanding Osmotic Pressure and Its Importance
The force exerted by water molecules when they move through a semi-permeable membrane is essential for maintaining the balance of fluids in living organisms. This pressure plays a crucial role in various biological processes, ensuring that cells remain hydrated and that vital nutrients and waste products are efficiently transported.
Key Factors Influencing Osmotic Pressure
- Concentration of Solutes: The more solutes present in a solution, the higher the osmotic pressure. This drives water to move towards areas with higher solute concentration to balance out the differences.
- Membrane Permeability: The ease with which water can pass through a membrane influences osmotic pressure. A membrane that allows only water molecules to pass will create a more noticeable pressure effect compared to one that permits other substances to pass as well.
- Temperature: Higher temperatures increase molecular movement, which in turn can increase osmotic pressure as water molecules move faster.
Importance in Biological Systems
This pressure is vital for the proper functioning of cells. In plants, it helps to maintain turgor pressure, which supports cell structure. In animals, it contributes to the regulation of blood volume and the exchange of nutrients and gases across cell membranes.
Understanding this pressure is essential in various medical and environmental fields. For example, in kidney function, it plays a role in filtering blood and regulating fluid balance. Additionally, osmotic pressure is a factor in maintaining the integrity of plant tissues and the overall health of organisms.
Common Diffusion Problems in Biology
In biological systems, the movement of substances across membranes is crucial for maintaining homeostasis and proper cellular function. However, various challenges can arise when this process is hindered or altered, leading to a range of issues that affect both cells and entire organisms. These disruptions can impact everything from nutrient uptake to waste removal, ultimately affecting the health and performance of biological systems.
Some of the most common difficulties include:
- Impaired Gas Exchange: When the movement of gases like oxygen and carbon dioxide across respiratory surfaces is reduced, it can lead to conditions like hypoxia or carbon dioxide buildup, severely affecting cellular respiration.
- Blocked Nutrient Transport: If the movement of essential nutrients into cells is obstructed, it can result in malnutrition and hinder cellular processes that rely on these nutrients.
- Water Imbalance: Improper movement of water across membranes can lead to dehydration or swelling of cells, causing structural damage or even cell death.
Addressing these issues often requires a deep understanding of how substances are transported within living systems, as well as how external factors such as temperature, pressure, and solute concentration can influence these processes.
Solving Osmosis Problems Step by Step
When faced with challenges related to the movement of water across a membrane, it is essential to approach the solution methodically. By breaking down the problem into manageable steps, you can accurately determine the direction of water flow, the impact on cell volume, and the potential changes in concentration across the membrane. These steps help in understanding how different factors influence cellular processes.
Step 1: Identify the Solute Concentrations
The first step in solving any challenge is to determine the concentration of solutes on both sides of the membrane. By understanding the relative concentrations of dissolved substances, you can predict which way water will move. Water always moves towards the area with the higher concentration of solutes in order to balance the concentrations.
Step 2: Understand the Effects on Cell Volume
Next, consider how the movement of water will affect the volume of the cell. If water moves into the cell, it will swell, while if water moves out, the cell will shrink. This is crucial for understanding the physical consequences of osmotic changes in living organisms.
Tip: When solving these types of challenges, remember that understanding the behavior of water in response to solute concentrations can provide insights into processes like hydration, nutrient absorption, and waste elimination in biological systems.
Effects of Temperature on Diffusion
Temperature plays a significant role in the rate at which particles move through a medium. As temperature increases, the energy of the molecules rises, leading to faster motion. This increase in kinetic energy directly influences how quickly substances spread and mix, affecting a wide range of processes in both living organisms and non-living systems.
Increased Kinetic Energy
When the temperature rises, the particles gain more kinetic energy, which causes them to move more rapidly. This leads to an acceleration in the movement of molecules from areas of higher concentration to lower concentration. The faster molecules move, the quicker they can spread out and reach equilibrium, which is a key factor in many biological processes.
Practical Implications
In biological systems, temperature influences processes like nutrient absorption, gas exchange, and waste removal. For instance, higher temperatures can enhance the efficiency of enzyme-catalyzed reactions that depend on the movement of molecules. However, excessive heat can also cause denaturation of proteins and damage cellular structures, highlighting the delicate balance between beneficial and harmful effects.
Note: Understanding how temperature affects molecular movement can help in areas like medicine, food preservation, and environmental science, where controlling the speed of these processes is crucial.
What Happens During Reverse Osmosis
Reverse osmosis is a process where water is forced through a semi-permeable membrane in the opposite direction of its natural flow. Unlike the usual movement, which occurs from areas of lower to higher solute concentration, this process uses pressure to push water from regions of higher solute concentration to areas with lower concentration. This results in the removal of impurities and contaminants from the water.
How It Works
During reverse osmosis, water is applied to one side of a membrane, which allows only water molecules to pass through while blocking larger molecules and ions. Pressure is applied to overcome the natural osmotic pressure, effectively “pushing” the water through the membrane. This separation results in purified water on one side and concentrated waste on the other.
Applications
Reverse osmosis is commonly used for water purification, desalination, and even in the food industry to concentrate or purify various liquids. By removing contaminants such as salts, heavy metals, and microorganisms, it helps provide clean, safe water for consumption and industrial use.
| Process | Result |
|---|---|
| Pressure applied to water | Water moves through membrane, leaving contaminants behind |
| Water molecules pass through | Pure water collected on one side |
| Contaminants blocked | Concentrated waste collected on other side |
Calculating Osmotic Pressure in Solutions
Osmotic pressure refers to the force exerted by a solvent as it moves through a semi-permeable membrane, driven by the concentration of solutes in the solution. This concept plays a key role in understanding how fluids move in biological systems and industrial applications. By calculating this pressure, one can predict the behavior of solutions under various conditions, including how much force is needed to prevent solvent movement across membranes.
Formula for Osmotic Pressure

The formula used to calculate osmotic pressure is derived from the ideal gas law and is given by:
π = i * M * R * T
Where:
- π = Osmotic pressure
- i = Van’t Hoff factor (number of particles the solute dissociates into)
- M = Molar concentration of the solution
- R = Ideal gas constant (0.0821 L·atm/mol·K)
- T = Temperature in Kelvin
Steps for Calculation
To calculate osmotic pressure, first, determine the molarity of the solution. Then, calculate the Van’t Hoff factor, which accounts for the dissociation of solute molecules in the solution. Finally, use the formula to calculate the osmotic pressure under the given conditions.
| Step | Action |
|---|---|
| 1 | Determine the concentration of the solution |
| 2 | Find the Van’t Hoff factor (i) |
| 3 | Use the formula to calculate osmotic pressure |
Example: For a 1.0 M NaCl solution at 298 K, the Van’t Hoff factor is 2 (since NaCl dissociates into two ions). Plugging values into the formula gives:
π = 2 * 1.0 * 0.0821 * 298 = 48.9 atm
This osmotic pressure can help in understanding processes such as filtration and the movement of water in biological systems.
Diffusion in Different States of Matter
The movement of particles from an area of high concentration to an area of low concentration occurs in all forms of matter. However, the rate and manner in which this process happens can differ depending on whether the substance is in a solid, liquid, or gas state. Understanding how particles interact in these states provides insight into various natural phenomena and industrial applications.
Gas Phase
In gases, particles are widely spaced and move freely in all directions. The high kinetic energy of gas molecules results in rapid mixing and spreading of substances. This is why the scent of perfume can be detected across a room almost immediately after it is sprayed.
- Particles have high mobility due to weak intermolecular forces.
- The process occurs quickly, as molecules are far apart and move rapidly.
- Temperature and pressure can significantly influence the rate of this process.
Liquid Phase

In liquids, particles are closer together than in gases, which results in a slower rate of movement. While still mobile, their interactions are more constrained, which means the spread of substances through liquids occurs more slowly than in gases. For example, when sugar is dissolved in water, it gradually spreads throughout the liquid.
- Intermolecular forces are stronger than in gases but weaker than in solids.
- Temperature increases tend to speed up the rate at which particles spread.
- The process takes longer due to closer particle interactions.
Solid Phase
In solids, particles are tightly packed and only vibrate in place. This restricts the movement of substances, making the process significantly slower compared to gases and liquids. An example can be seen in the slow migration of atoms in a metal over long periods, which is known as solid-state diffusion.
- Particle movement is minimal due to strong intermolecular forces.
- Diffusion in solids occurs very slowly unless the material is heated or altered chemically.
- The process is highly dependent on the nature of the solid material.
Overall, the ability of particles to move and mix varies greatly with the state of matter, which explains why certain processes happen faster or slower depending on whether they are occurring in a solid, liquid, or gas. Understanding these differences is key in fields such as chemistry, biology, and materials science.
Applications of Osmosis in Medicine
The principle of movement of water through a semi-permeable barrier is widely utilized in the medical field, offering solutions to numerous health-related issues. This process plays a key role in various therapeutic treatments and diagnostic methods, helping to maintain proper fluid balance in the body and to enhance drug delivery systems.
IV Fluid Administration
In medical treatment, the use of intravenous (IV) fluids is a common application where the movement of water across membranes is crucial. The careful selection of fluid composition, such as saline or glucose solutions, is designed to mimic the body’s natural processes and maintain homeostasis by balancing the fluid levels between the blood and tissues.
- Hydration therapy relies on the correct osmotic balance to ensure proper cell function.
- IV solutions with varying concentrations help restore lost electrolytes and fluids.
- Administration of osmotic fluids helps in cases of dehydration, shock, and surgery recovery.
Dialysis Treatment
Another key application is in dialysis, a treatment for kidney failure. This procedure involves a semi-permeable membrane that allows toxins and waste products to pass from the bloodstream into a dialysis solution. The process is based on the natural movement of water and solutes, ensuring that harmful substances are removed while maintaining a balance of essential nutrients in the body.
- Dialysis uses osmotic pressure to filter waste from the blood when the kidneys cannot perform this function.
- The movement of fluids through membranes ensures the removal of excess salts, urea, and toxins.
- It helps to regulate the body’s fluid balance and electrolyte levels in patients with kidney disease.
Drug Delivery Systems
The movement of substances across membranes is also crucial in the development of controlled drug delivery systems. Osmotic pressure can be used to design drug capsules that release medication gradually over time, ensuring steady therapeutic effects. This controlled release minimizes side effects and optimizes drug efficiency, especially in long-term treatments.
- Osmotic-driven pumps are designed to deliver medication in a regulated manner.
- This method is commonly used for insulin delivery in diabetic patients.
- It allows for the precise release of drugs like painkillers or cancer treatments, improving patient comfort and outcomes.
These examples demonstrate how the fundamental process of water movement through membranes plays a critical role in various medical treatments. Understanding this principle enhances healthcare practices and leads to more effective solutions in both acute and chronic medical conditions.
Why Diffusion is Essential for Cells
The movement of substances across cellular membranes is a vital process that supports numerous biological functions. This movement allows cells to exchange gases, nutrients, and waste products with their environment, maintaining a balance that is crucial for survival. Without this mechanism, cells would be unable to perform basic life-sustaining activities such as energy production and waste elimination.
Role in Nutrient Uptake
One of the key functions of this natural process is the uptake of essential nutrients from the surrounding environment. The cells rely on the movement of nutrients like oxygen, glucose, and minerals to maintain metabolic activities. This ensures that cells have the resources needed for energy production and growth.
- Oxygen moves from areas of higher concentration, such as blood vessels, into cells where it is needed for respiration.
- Glucose and other nutrients flow into cells to support cellular functions and energy production.
- Vitamins and minerals required for biochemical processes enter the cell through this natural mechanism.
Elimination of Waste Products
In addition to nutrient uptake, this process also helps to remove waste products generated within the cell. By facilitating the movement of carbon dioxide, urea, and other metabolic byproducts out of the cell, it ensures that the internal environment remains clean and conducive to optimal cellular function.
- Waste products such as carbon dioxide are released from cells into the bloodstream, where they are carried away for elimination.
- Urea, a byproduct of protein metabolism, is transported to the kidneys for excretion.
- This mechanism maintains the internal stability and health of the cell.
Maintaining Homeostasis
The continuous movement of substances into and out of cells is also essential for maintaining a stable internal environment, a process known as homeostasis. By regulating the concentrations of ions, water, and other molecules, cells are able to maintain their shape, volume, and internal chemical balance.
- Electrolyte balance is crucial for nerve and muscle function, which is regulated through this process.
- Water movement helps to control the hydration levels and pressure within the cell.
- Maintaining proper ion concentrations is essential for electrical signaling within cells.
In summary, the movement of substances across cell membranes is essential for survival. It enables the cell to take in nutrients, eliminate waste, and maintain a stable internal environment, ensuring that cellular processes continue to run smoothly.
Osmosis in Animal Cells
The movement of water across the membrane of animal cells plays a crucial role in maintaining cellular function. This process ensures that cells stay hydrated, maintain their shape, and operate efficiently. It is essential for the regulation of fluid balance within the cell and its environment, which directly affects the cell’s ability to perform vital tasks.
Water Movement in Animal Cells
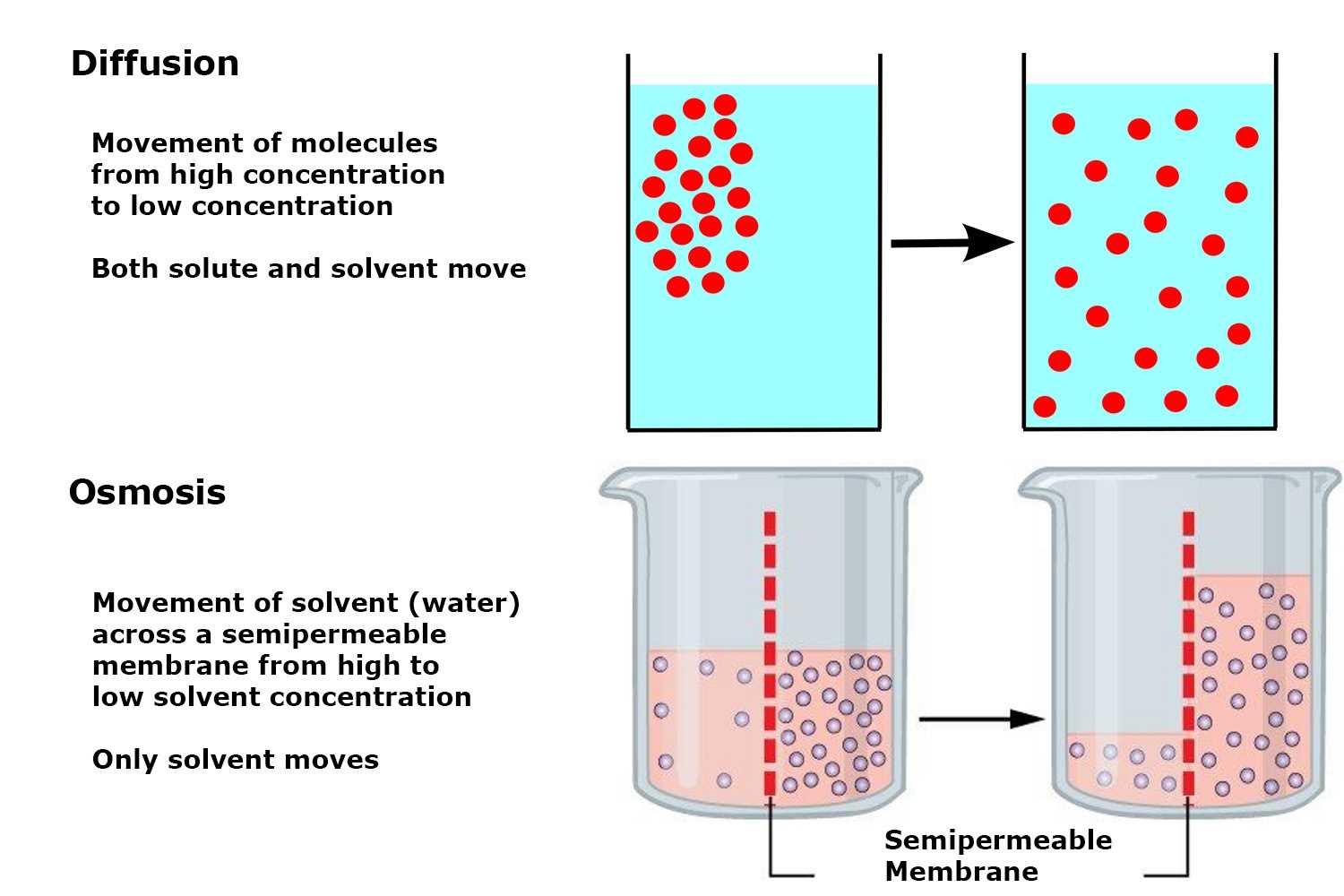
Water enters and exits animal cells through the selective permeability of the cell membrane. The direction of water flow is determined by the concentration gradient of water molecules between the inside of the cell and the surrounding solution. This movement is vital for maintaining a stable internal environment.
- If the surrounding fluid has a higher concentration of water than the cell’s interior, water flows into the cell, keeping it hydrated.
- If the surrounding fluid has a lower concentration of water, water flows out of the cell, which can lead to dehydration.
- Cells are constantly adjusting their internal conditions to ensure proper function and structure.
Effects on Cell Shape and Function
Changes in the water content of animal cells can affect their shape and overall health. The ability of the cell to regulate water balance is essential to prevent harmful conditions such as swelling or shrinkage. Both extreme cases can lead to dysfunction or cell death.
- In a hypertonic environment (higher solute concentration outside the cell), water leaves the cell, causing it to shrink. This can impair cell function.
- In a hypotonic environment (lower solute concentration outside the cell), water enters the cell, causing it to swell. If the pressure becomes too great, the cell may burst.
- Isotonic conditions, where the water concentration is the same inside and outside the cell, maintain the cell’s size and function.
In summary, maintaining proper water balance is essential for the health of animal cells. The movement of water through the cell membrane ensures that cells can carry out their functions while avoiding potential damage from changes in cell volume.
Common Mistakes in Diffusion and Osmosis Problems
When working through exercises involving the movement of substances across membranes, several common errors can arise. These mistakes often stem from misunderstandings of key concepts, incorrect application of principles, or failure to carefully analyze the situation. Recognizing these common pitfalls can help improve accuracy and understanding.
Misunderstanding the Direction of Movement
A frequent mistake is misinterpreting the direction of movement of substances. In many cases, individuals fail to correctly determine whether a substance moves into or out of the cell or system based on concentration gradients.
- Confusing which way water moves in relation to solute concentrations is a common issue. Water always moves from areas of lower solute concentration to areas of higher concentration, but it’s important to carefully assess the system’s conditions to predict the direction correctly.
- Another mistake is not recognizing that the movement of particles is passive unless energy is required. This often leads to incorrect assumptions about active transport when it’s not necessary.
Overlooking the Role of the Membrane
The selective permeability of membranes is essential to understanding the movement of substances. Neglecting how certain molecules pass through membranes while others are restricted can lead to errors when solving related exercises.
- Assuming all particles can pass through the membrane freely is a mistake. Many molecules require specific channels or carriers to move across membranes, which is crucial when determining the behavior of certain substances in different conditions.
- Ignoring the properties of the membrane, such as size restrictions or the need for energy, can also result in misunderstanding how substances distribute across compartments.
Avoiding these mistakes requires careful attention to the key principles of concentration gradients, membrane properties, and passive versus active transport. By understanding the details and applying them correctly, it’s easier to approach these types of exercises with greater precision.