
In the world of chemistry, reactions are constantly adjusting to maintain balance. When conditions such as temperature, pressure, or concentration change, the system responds by shifting its state to restore equilibrium. This fundamental concept plays a crucial role in both theoretical studies and real-world applications. By understanding how different factors influence chemical behavior, we can predict and control reaction outcomes more effectively.
In practical experiments, these shifts can be observed and analyzed, providing valuable insights into how substances interact and transform. By manipulating variables, scientists can study the underlying principles that govern these reactions, gaining a deeper understanding of chemical dynamics. This knowledge is not only vital for research but also essential for various industries, from pharmaceuticals to environmental science.
Equilibrium reactions are an essential part of many processes, and the ability to predict their behavior is key to advancing scientific knowledge. Whether it’s improving manufacturing techniques or developing new materials, the concepts derived from studying reaction shifts remain at the forefront of innovation.
Applications of Le Chatelier’s Principle in Labs

In scientific investigations, understanding how chemical systems respond to changes is fundamental. By altering factors such as pressure, temperature, or the concentration of reactants, researchers can observe how these changes affect the direction of chemical reactions. This knowledge is essential for controlling and optimizing reactions in both industrial and academic settings. It provides valuable insights into reaction rates, product yields, and the efficiency of processes.
Experiments focused on reaction equilibrium typically involve a variety of conditions that simulate real-world applications. In a controlled environment, shifts in the system can be carefully observed, allowing researchers to test various hypotheses and predictions. This type of experimentation helps clarify the mechanisms behind many reactions that occur in nature or industry.
- Temperature Effects: Heating or cooling a system can cause shifts that help identify reaction pathways and improve yields.
- Concentration Adjustments: Changing the amount of reactants or products helps determine how equilibrium is maintained and where reactions favor.
- Pressure Influence: In reactions involving gases, altering pressure can have significant effects, making it essential for processes like ammonia synthesis.
- Using Catalysts: Adding catalysts can accelerate reactions and provide insight into how systems reach equilibrium faster.
These experiments are invaluable in fields ranging from pharmaceuticals to environmental science. In many industrial processes, maximizing efficiency and minimizing waste are top priorities. By applying these concepts, chemists and engineers can improve reaction yields, reduce energy consumption, and minimize the environmental impact of chemical processes.
Understanding Chemical Equilibrium Concepts
Chemical reactions often reach a point where the rate of the forward reaction equals the rate of the reverse reaction. This state is known as equilibrium, where the concentrations of reactants and products remain constant over time. It is important to note that this does not mean the reactions stop; rather, the forward and reverse processes occur at equal rates, keeping the system balanced.
At equilibrium, the system has reached a dynamic balance where the ratio of products to reactants stays constant. However, this balance can shift when external conditions, such as temperature, pressure, or concentration, are changed. Understanding how and why these shifts occur is crucial for predicting the outcome of various chemical processes, from industrial manufacturing to environmental reactions.
In real-world scenarios, equilibrium is a central concept in fields like chemistry, biology, and engineering. The ability to manipulate and control equilibrium is key to improving the efficiency of chemical reactions in industries such as pharmaceuticals, energy production, and food processing.
Impact of Temperature on Equilibrium Shifts
Temperature plays a significant role in determining the direction in which a chemical reaction will proceed. When the temperature of a system is altered, the system adjusts in a way that either absorbs or releases heat to counteract the change. This shift can either favor the production of reactants or products, depending on whether the reaction is exothermic or endothermic.
In an exothermic reaction, where heat is released, increasing the temperature typically causes the system to shift towards the reactants, as the system tries to absorb the added heat. Conversely, in an endothermic reaction, where heat is absorbed, raising the temperature will shift the equilibrium towards the products, as the system seeks to release the excess heat. Understanding this dynamic is crucial for controlling reaction rates and optimizing product yields in various chemical processes.
Temperature control is often employed in industrial settings to ensure reactions occur at the desired rate and to maximize efficiency. By adjusting the temperature, chemists can influence the equilibrium position, making it easier to achieve specific outcomes in processes such as chemical synthesis, energy production, and even environmental remediation.
Effect of Pressure on Chemical Reactions
Pressure is an important factor that can influence the direction of chemical reactions, particularly those involving gases. When the pressure of a system is altered, the equilibrium position can shift to either favor the reactants or the products, depending on the number of gas molecules involved in the reaction. Increasing pressure generally favors the side of the reaction with fewer gas molecules, while decreasing pressure tends to favor the side with more gas molecules.
This principle is especially relevant in industrial processes that involve gaseous reactants, such as the synthesis of ammonia or the production of chemicals in high-pressure reactors. By manipulating pressure, chemists can control the rate of reaction and optimize yields for specific products, making it an essential tool in large-scale chemical manufacturing.
| Reaction | Effect of Increased Pressure |
|---|---|
| 2 N2 + 3 H2 ⇌ 2 NH3 | Favors the production of ammonia (fewer gas molecules) |
| CO2 + C ⇌ 2 CO | Favors the side with more gas molecules (products) |
| 2 SO2 + O2 ⇌ 2 SO3 | Favors the production of sulfur trioxide (fewer gas molecules) |
In conclusion, pressure plays a crucial role in controlling the outcomes of chemical reactions, particularly in processes where gases are involved. Understanding how pressure affects equilibrium allows scientists and engineers to design more efficient processes and achieve desired results with greater precision.
Concentration Changes and Equilibrium Responses
Changes in the concentration of reactants or products in a chemical reaction can have a profound effect on the position of equilibrium. When the concentration of a substance is increased or decreased, the system adjusts to minimize the disturbance, shifting the reaction towards the side that counteracts the change. This response allows the system to restore balance and maintain a state of equilibrium.
Increasing the concentration of reactants will generally cause the system to shift towards the products, while increasing the concentration of products will push the reaction towards the reactants. This behavior is a key feature of many chemical processes and is particularly useful in industries where maximizing yield is a priority.
- Increasing Reactant Concentration: Shifts equilibrium to favor product formation.
- Increasing Product Concentration: Shifts equilibrium towards the reactants to reduce product concentration.
- Decreasing Reactant Concentration: Shifts equilibrium to favor more reactants.
- Decreasing Product Concentration: Shifts equilibrium to favor more product formation.
By carefully controlling the concentration of various substances, chemists can manipulate reaction rates and improve the efficiency of chemical processes. This concept is widely used in industries such as pharmaceuticals, where the precise production of compounds is essential for quality control and cost efficiency.
Real-World Examples of Le Chatelier’s Principle
The concept of equilibrium shifts is not just a theoretical idea but plays a critical role in many real-world chemical processes. In various industries, understanding how reactions adjust to changes in conditions such as temperature, pressure, and concentration allows for improved efficiency and optimized outcomes. By applying this knowledge, scientists and engineers can design processes that are both cost-effective and sustainable.
One of the most notable examples is the Haber process, used for the industrial synthesis of ammonia. In this process, nitrogen and hydrogen gases react to form ammonia, and by adjusting the temperature and pressure, producers can maximize the yield. Increasing pressure shifts the reaction towards the production of ammonia, while decreasing temperature favors the reverse reaction. This balance between temperature and pressure is carefully controlled to achieve optimal production rates.
Another example is carbon dioxide removal in chemical plants. By altering the concentration of CO2 in the system, plants can drive the reactions that absorb or release the gas, maintaining the desired balance in the atmosphere. This principle is also applied in biological processes, such as photosynthesis, where the concentration of carbon dioxide in plant cells can influence the rate of the reaction, thus ensuring the plant’s growth and survival.
In environmental science, the principles behind equilibrium shifts are used to manage pollution control systems, where changes in temperature or pressure can optimize chemical reactions designed to neutralize harmful substances. From manufacturing to medicine, understanding how systems respond to changes ensures more efficient processes that benefit both the economy and the environment.
Using Le Chatelier’s Principle in Industrial Chemistry
In industrial chemistry, understanding how chemical reactions respond to changes in their environment is crucial for optimizing production processes. By manipulating conditions such as temperature, pressure, and the concentration of reactants and products, manufacturers can shift reactions in a way that maximizes yield, reduces costs, and improves efficiency. This knowledge is applied in various chemical industries to ensure that processes run smoothly and economically.
For example, in the production of ammonia through the Haber process, conditions are carefully adjusted to shift the reaction towards the production of ammonia. By increasing pressure, the reaction favors the formation of ammonia, as fewer gas molecules are present on the product side. Similarly, by controlling temperature, producers can balance the rate of reaction with energy efficiency, achieving higher yields while minimizing energy consumption.
In other industrial processes, such as the production of sulfuric acid, reactions can be manipulated by changing the concentration of reactants. For instance, adding more oxygen in the synthesis of sulfur trioxide can drive the reaction forward, increasing the amount of sulfuric acid produced. The ability to control these variables is a fundamental aspect of chemical engineering and industrial chemistry, making processes more efficient and sustainable.
By applying the concepts of reaction equilibrium, industries can reduce waste, improve product quality, and ensure that reactions are completed at the optimal rate. This approach not only saves time and money but also plays a vital role in meeting the growing demand for chemicals and materials in various sectors, from energy to pharmaceuticals.
Le Chatelier’s Principle in Acid-Base Reactions

In acid-base reactions, equilibrium plays a crucial role in determining the direction of the reaction and the concentrations of the reactants and products. When the concentration of an acid or a base is altered, the reaction shifts to restore balance, either producing more acid or base to counteract the change. This principle is important for controlling pH levels in both laboratory and industrial processes, where maintaining a specific balance is often essential.
For example, in the reaction between acetic acid and water, if the concentration of acetic acid is increased, the reaction will shift to produce more hydrogen ions and acetate ions in response. Similarly, if the concentration of acetate ions is raised, the reaction will shift towards the left, favoring the formation of more acetic acid. This dynamic shift helps maintain equilibrium and pH stability in various environments.
- Increasing Acid Concentration: Shifts the reaction towards more dissociation, producing more hydrogen ions.
- Increasing Base Concentration: Shifts the reaction towards the formation of more water, reducing hydrogen ion concentration.
- Adding a Salt: Can affect the concentration of ions in the solution, leading to shifts in the reaction equilibrium.
- Changing Temperature: In exothermic acid-base reactions, increasing temperature can shift the equilibrium towards more dissociation of the acid.
This shifting equilibrium is not only observed in simple acid-base reactions but is also vital in more complex systems, such as buffer solutions, which are designed to resist changes in pH. In these systems, adding small amounts of acids or bases will not drastically affect the pH, thanks to the equilibrium adjustments that occur.
In industrial processes like the production of fertilizers or pharmaceuticals, understanding how acid-base reactions respond to changes in concentration helps optimize reactions, reduce waste, and increase product yield. The ability to control these shifts is vital for maintaining the desired chemical properties and achieving the most efficient reaction outcomes.
Lab Experiments Demonstrating Equilibrium Shifts

In scientific experiments, observing how chemical systems respond to changes in concentration, temperature, or pressure is essential for understanding dynamic equilibria. These changes can cause the system to shift its composition to restore balance. Several common experiments illustrate how these shifts occur, providing valuable insights into the principles behind equilibrium behavior.
Effect of Temperature on Equilibrium
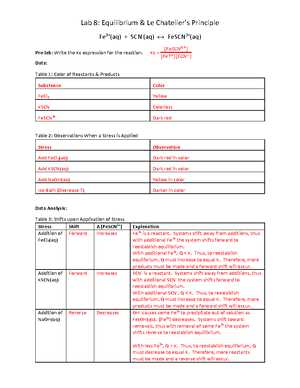
One classic experiment involves the reversible reaction between iron(III) chloride and thiocyanate ions to form a red complex. By heating or cooling the system, the equilibrium position can be altered. An increase in temperature typically shifts the equilibrium towards the endothermic direction, resulting in a change in color as the concentration of products increases or decreases.
| Temperature | Equilibrium Shift | Resulting Color |
|---|---|---|
| High | Towards products (endothermic) | Dark red |
| Low | Towards reactants (exothermic) | Light red |
Concentration Changes and Shifts
Another experiment demonstrates the effect of concentration changes on equilibrium. The reaction between hydrogen and iodine gases to form hydrogen iodide is reversible. By increasing the concentration of one of the reactants, the system adjusts to produce more products. Similarly, if the concentration of hydrogen iodide is increased, the reaction shifts towards the reactants.
| Change in Concentration | Equilibrium Shift |
|---|---|
| Increase in hydrogen | Shift towards products |
| Increase in hydrogen iodide | Shift towards reactants |
These experiments are fundamental for visualizing how equilibrium systems respond to external changes. Through controlled manipulation of conditions, scientists gain a deeper understanding of the dynamic nature of chemical reactions, paving the way for more efficient industrial processes and advanced research.
Observing Le Chatelier’s Principle in Action
In many scientific experiments, the response of a chemical system to changes in its environment provides a clear demonstration of how reactions adjust to maintain equilibrium. By altering variables such as temperature, pressure, or the concentration of reactants or products, one can visually witness the system’s tendency to shift in a way that counteracts the change. These observations highlight the dynamic nature of chemical reactions and provide a deeper understanding of reaction mechanisms.
One notable experiment involves the reversible reaction between cobalt chloride and water. In the presence of heat, the equilibrium shifts to produce a more concentrated solution of cobalt(II) chloride, turning the solution from pink to blue. Conversely, cooling the solution causes the reaction to shift back, restoring the pink color as cobalt chloride precipitates out. This experiment vividly illustrates how changes in temperature influence the direction of the reaction, favoring the endothermic or exothermic pathways depending on the conditions.
Similarly, by manipulating the pressure in reactions involving gases, such as the synthesis of ammonia, one can observe how a change in pressure causes a shift in the equilibrium position. An increase in pressure often favors the side of the reaction with fewer gas molecules, driving the system towards products or reactants depending on the stoichiometry of the reaction.
These experiments are not only fundamental for understanding chemical equilibria but are also valuable tools in both educational and industrial settings, where controlling and optimizing reaction conditions is key to achieving desired outcomes. By observing how chemical systems respond to external influences, scientists and engineers can refine processes for greater efficiency and effectiveness.
Le Chatelier’s Principle and Levers in Reactions

In chemical reactions, the concept of balance can be compared to the function of a lever. Just as a lever shifts under force to achieve equilibrium, chemical reactions adjust to external changes in order to restore a state of balance. When one part of a reaction is altered–whether by changing temperature, pressure, or concentration–the system reacts by shifting to oppose that change, much like a lever moving to counterbalance applied forces.
The Lever Analogy in Chemical Equilibrium
Consider a lever with a fulcrum in the middle. If a force is applied on one side, the lever moves in the opposite direction to maintain balance. In a chemical system, when one component of the reaction is manipulated–such as increasing the concentration of a reactant–the system “moves” to adjust, either by increasing or decreasing the concentration of products to restore equilibrium.
Shifting Reactions Under External Influences
Just as the position of a lever can be influenced by where the force is applied, the direction of a reaction can be influenced by external factors. For instance, increasing pressure in a gas-phase reaction often shifts the equilibrium towards the side with fewer gas molecules, as the system “pushes” back to relieve the stress, akin to a lever shifting to maintain balance under changing forces.
This analogy helps visualize the dynamic nature of chemical reactions, emphasizing how systems are constantly adjusting to maintain equilibrium. Understanding these shifts allows scientists to manipulate reaction conditions effectively, whether in a laboratory or industrial setting, to optimize outcomes and ensure reactions proceed efficiently.
Common Errors in Le Chatelier’s Principle Labs
When conducting experiments related to the shifting of chemical reactions under various conditions, several common mistakes can hinder accurate observations and interpretations. These errors often stem from improper manipulation of variables, misinterpretation of results, or overlooked external factors that influence the equilibrium. Recognizing these mistakes is crucial for ensuring reliable experimental outcomes and understanding the fundamental concepts involved.
Misunderstanding the Effect of Temperature
One of the most frequent mistakes in these types of experiments is misjudging how temperature changes affect the direction of a reaction. In some cases, students may assume that heating a system will always favor the forward reaction, while cooling always favors the reverse. However, depending on whether the reaction is endothermic or exothermic, the effect of temperature may differ. A lack of attention to the nature of the reaction can lead to incorrect conclusions about the system’s behavior.
Incorrect Pressure Adjustments
Another common error occurs when altering the pressure in reactions involving gases. Many students may not realize that pressure changes affect reactions differently depending on the number of gas molecules on each side of the equation. Increasing pressure will shift the equilibrium towards the side with fewer gas molecules, but if both sides have the same number, pressure changes will have no significant effect. Failing to account for this can lead to inaccurate predictions of how the system will respond to pressure changes.
These common errors can lead to misconceptions and skewed data, making it essential for students to approach these experiments with a clear understanding of the factors at play. Careful attention to the details of the system and the conditions applied will lead to more accurate results and a better grasp of chemical equilibrium dynamics.
Applications in Environmental Chemistry Studies
In environmental chemistry, the understanding of how chemical reactions respond to changes in conditions plays a vital role in addressing issues such as pollution, climate change, and resource management. By studying how chemical systems shift in response to factors like temperature, pressure, and concentration, researchers can predict and manipulate reactions that affect ecosystems and atmospheric processes. These insights help to design more efficient ways of managing industrial emissions, waste treatment, and sustainable practices.
One key example is in the study of carbon dioxide in the atmosphere, which influences global climate patterns. By understanding how the concentration of gases affects equilibrium in natural systems, scientists can predict how shifts in the environment might impact the carbon cycle and global warming. Similarly, in water treatment processes, adjusting the chemical equilibrium can help to remove harmful contaminants, ensuring cleaner water for both human use and environmental health.
These studies also extend to the development of renewable energy technologies, where understanding reaction shifts can improve the efficiency of processes such as biofuel production or hydrogen storage. By applying these concepts, environmental chemists are better equipped to address complex environmental challenges, ensuring a more sustainable and balanced relationship between human activities and the planet’s natural systems.
Le Chatelier’s Principle in Biochemical Processes
In biochemistry, the concept of how systems adjust to external changes is fundamental to understanding a variety of processes that occur in living organisms. These processes involve dynamic reactions that maintain life, such as enzyme catalysis, metabolism, and cellular respiration. When environmental conditions such as temperature, pH, or substrate concentration change, the reactions within biological systems shift to maintain equilibrium, ensuring the stability necessary for life.
Enzyme Activity and Reaction Shifts

One of the most well-known examples of this dynamic adjustment is seen in enzyme-catalyzed reactions. Enzymes are biological catalysts that accelerate chemical reactions within cells. The activity of enzymes can be influenced by changes in factors such as temperature or pH. For instance, increasing the temperature can speed up a reaction, but if the temperature exceeds a certain threshold, it may cause the enzyme to denature and lose its functionality. This shift in the reaction can be understood through the principles of equilibrium, where enzymes adapt to conditions to optimize reaction rates.
Metabolic Pathways and Homeostasis
Metabolic pathways within cells, including the breakdown of glucose or the synthesis of amino acids, also rely on this shifting balance. Cells must maintain homeostasis, the stable internal environment that allows biochemical processes to function efficiently. For example, if there is an increase in the concentration of one substance in a metabolic pathway, the system may adjust by shifting the equilibrium toward the breakdown or utilization of that substance, thus maintaining balance within the cell.
- Temperature Changes: Can increase reaction rates but may also denature enzymes beyond optimal conditions.
- pH Shifts: Changes in acidity or alkalinity can influence enzyme activity and the direction of reactions.
- Substrate Concentration: A higher concentration can drive reactions forward, while lower levels may slow down the process.
Understanding how biochemical processes respond to external changes allows researchers to manipulate conditions in laboratory settings to study disease mechanisms, drug interactions, and even design therapeutic interventions. By applying these concepts, scientists can predict how the body will react to changes in its environment and how it can be influenced for therapeutic purposes.
Laboratory Equipment for Studying Equilibrium

In the study of chemical reactions and their balance, a variety of specialized equipment is used to observe and manipulate systems at equilibrium. These tools allow researchers to monitor variables such as concentration, temperature, and pressure, which can influence the direction of a reaction. Using the appropriate instruments ensures precise measurements and accurate results when studying equilibrium shifts in both educational and professional research settings.
Measuring Temperature and Pressure
Temperature and pressure are critical factors in determining the direction of a reaction at equilibrium. To study these factors, equipment such as thermometers and pressure sensors is commonly employed. A thermometer, especially one with a high level of precision, helps track any temperature fluctuations that may affect the reaction rates. Similarly, pressure sensors and manometers are used to measure the pressure within closed systems, such as gas reactions. Both temperature and pressure can influence the equilibrium position, and accurate measurement of these parameters is essential for understanding how the system responds to changes.
Monitoring Concentration Changes
Changes in the concentration of reactants or products are often the primary focus when studying equilibrium systems. To monitor these variations, various methods and equipment can be used. Spectrophotometers are often employed to measure the absorbance of light by a solution, which can be correlated with the concentration of specific ions or molecules. Another useful tool is the pH meter, which is used to determine the acidity or alkalinity of a solution. The concentration of hydrogen ions in the solution can affect the position of equilibrium in many reactions, particularly acid-base reactions.
- Thermometers: Essential for monitoring temperature fluctuations that influence reaction rates.
- Pressure Sensors: Measure the pressure within reaction vessels to understand the effect of pressure changes on equilibrium.
- Spectrophotometers: Used to track concentration changes by measuring the absorption of light.
- pH Meters: Help determine changes in the acidity or alkalinity of the solution, influencing reaction dynamics.
By utilizing this array of instruments, researchers are able to manipulate and observe equilibrium systems, allowing them to test hypotheses and gather data that contribute to a deeper understanding of chemical behavior. These tools not only enhance accuracy but also ensure that the results are reproducible under controlled conditions, a critical aspect of scientific inquiry.
Future Research Directions in Le Chatelier’s Applications
The study of chemical equilibrium continues to evolve, offering new opportunities to explore how external factors influence reaction dynamics. Researchers are increasingly focusing on advancing the understanding of equilibrium shifts through novel methodologies and technologies. As science progresses, future research will likely expand on both theoretical and practical applications, opening new frontiers in fields such as industrial chemistry, environmental science, and biochemistry.
Exploring the Role of Nanotechnology
One promising area of research lies in the intersection of equilibrium systems and nanotechnology. The unique properties of nanoparticles, including their large surface area and reactivity, may significantly impact the way reactions at equilibrium behave. Investigating how nanoparticles interact with reactants and products could lead to new catalysts or methods for controlling equilibrium positions more efficiently. Future studies may also explore how nanoparticles influence equilibrium in complex biochemical pathways, potentially leading to breakthroughs in drug delivery systems or environmentally sustainable processes.
Advanced Computational Modeling
With advancements in computational chemistry, researchers are developing more sophisticated models to predict the behavior of equilibrium systems under various conditions. These models integrate quantum mechanics and molecular dynamics simulations to simulate reactions more accurately than ever before. In the coming years, these tools may provide unprecedented insights into the subtle changes in molecular interactions that drive equilibrium shifts. This will be particularly valuable in predicting outcomes in industrial applications, where precise control over reaction rates and product yields is critical.
As these research directions continue to grow, the focus on understanding and applying equilibrium shifts will have far-reaching consequences across a range of industries. Innovations in material science, sustainable chemistry, and biomedical applications are just a few examples of how the ongoing study of reaction balance could shape the future of scientific research and technology.