The arrangement of electrons in an atom plays a crucial role in determining its properties and behavior. By using a specific set of rules and notations, scientists can describe how electrons occupy various energy levels and orbitals around the nucleus. This process is essential for understanding atomic interactions, bonding, and the overall structure of matter.
One common way to represent this arrangement is through a shorthand notation that simplifies the description of electron placement in atoms. This notation provides a quick and effective way to communicate the distribution of electrons without needing to list each individual electron. Instead, it uses a system of numbers and letters that indicate the specific orbitals and energy levels that electrons occupy.
By breaking down this notation, it becomes easier to identify elements, understand their properties, and predict how they might interact in different chemical reactions. In this article, we will explore the method of translating electron arrangements into simplified forms, offering a clearer picture of atomic structure.
Understanding Electron Configuration Basics
The way electrons are arranged within an atom is a fundamental aspect of chemistry. These arrangements determine how atoms interact with each other and form compounds. Atoms consist of a nucleus surrounded by electrons, and the specific arrangement of these electrons defines many of the atom’s characteristics, including its chemical reactivity.
To describe this arrangement, scientists use a set of rules that explain how electrons fill various energy levels, or orbitals. These rules are based on quantum mechanics and dictate the order in which electrons occupy different regions around the nucleus. The notation used to represent this organization offers a quick way to visualize and communicate the electron distribution within an atom.
The primary objective of understanding this arrangement is to predict an element’s behavior in reactions and its position in the periodic table. By knowing how electrons are positioned, it is possible to anticipate how atoms will bond, share, or transfer electrons during chemical interactions.
What is Electron Configuration?
Electron configuration refers to the arrangement of electrons within an atom’s orbitals. These configurations are key to understanding how atoms interact, bond, and react with other elements. The way electrons are distributed determines the chemical properties of an element, including its reactivity and its ability to form stable compounds.
Basic Principles of Electron Configuration
Electrons fill orbitals in a specific order, based on energy levels. Each orbital can hold a certain number of electrons, and the electrons are distributed according to principles derived from quantum mechanics. The configuration reflects the most stable arrangement, where electrons occupy the lowest energy states first.
Rules for Electron Distribution
Several key principles govern how electrons are arranged in an atom. These rules help to determine the electron configuration for any given element:
| Rule | Description |
|---|---|
| Aufbau Principle | Electrons fill lower energy orbitals before moving to higher energy orbitals. |
| Pauli Exclusion Principle | Each orbital can hold a maximum of two electrons with opposite spins. |
| Hund’s Rule | Electrons occupy degenerate orbitals singly before pairing up. |
By following these rules, scientists can determine the most likely electron arrangement for any element, providing insight into its chemical behavior and bonding potential. This knowledge is fundamental to understanding atomic interactions across the periodic table.
The Structure of an Atom Explained
An atom is the basic unit of matter, consisting of a dense nucleus surrounded by a cloud of negatively charged particles. This structure forms the foundation for all chemical substances, determining how atoms bond, interact, and behave in different environments. At the center of the atom is the nucleus, which contains protons and neutrons, while electrons orbit the nucleus in specific regions called orbitals.
The protons within the nucleus carry a positive charge, and the number of protons determines the element’s identity. Neutrons, which have no charge, help stabilize the nucleus. Electrons, on the other hand, are much lighter than protons and move around the nucleus at various energy levels. These electrons are essential in forming bonds with other atoms and are responsible for the chemical properties of elements.
The arrangement of these particles is crucial to understanding how atoms interact. The energy levels or shells around the nucleus play a significant role in determining the stability of an atom and its potential for bonding with other atoms. The more electrons an atom has in its outermost shell, the more reactive it may be in chemical processes.
How Electrons Fill Atomic Orbitals
Electrons in an atom occupy distinct regions known as orbitals, which are areas where they are most likely to be found. The process of how electrons fill these orbitals is governed by specific principles that ensure the most stable configuration. This arrangement influences an atom’s energy, its chemical reactivity, and its ability to bond with other atoms.
Order of Orbital Filling
Electrons fill orbitals in a defined order, starting with the lowest energy levels. This is known as the Aufbau principle. Orbitals within a given energy level are filled before electrons move to higher levels. The order follows a specific pattern based on the energy and spatial distribution of the orbitals.
Energy Levels and Subshells
Each energy level in an atom contains a specific set of orbitals, grouped into subshells labeled s, p, d, and f. The s subshell has one orbital, the p subshell has three, the d subshell contains five, and the f subshell contains seven. Electrons first fill the orbitals in the lowest energy subshells, following the principle of least energy to achieve stability.
As the number of electrons increases, they fill orbitals according to these rules, gradually moving from the innermost shells to the outermost regions. The distribution of electrons across these orbitals ultimately dictates an atom’s properties and its behavior in chemical reactions.
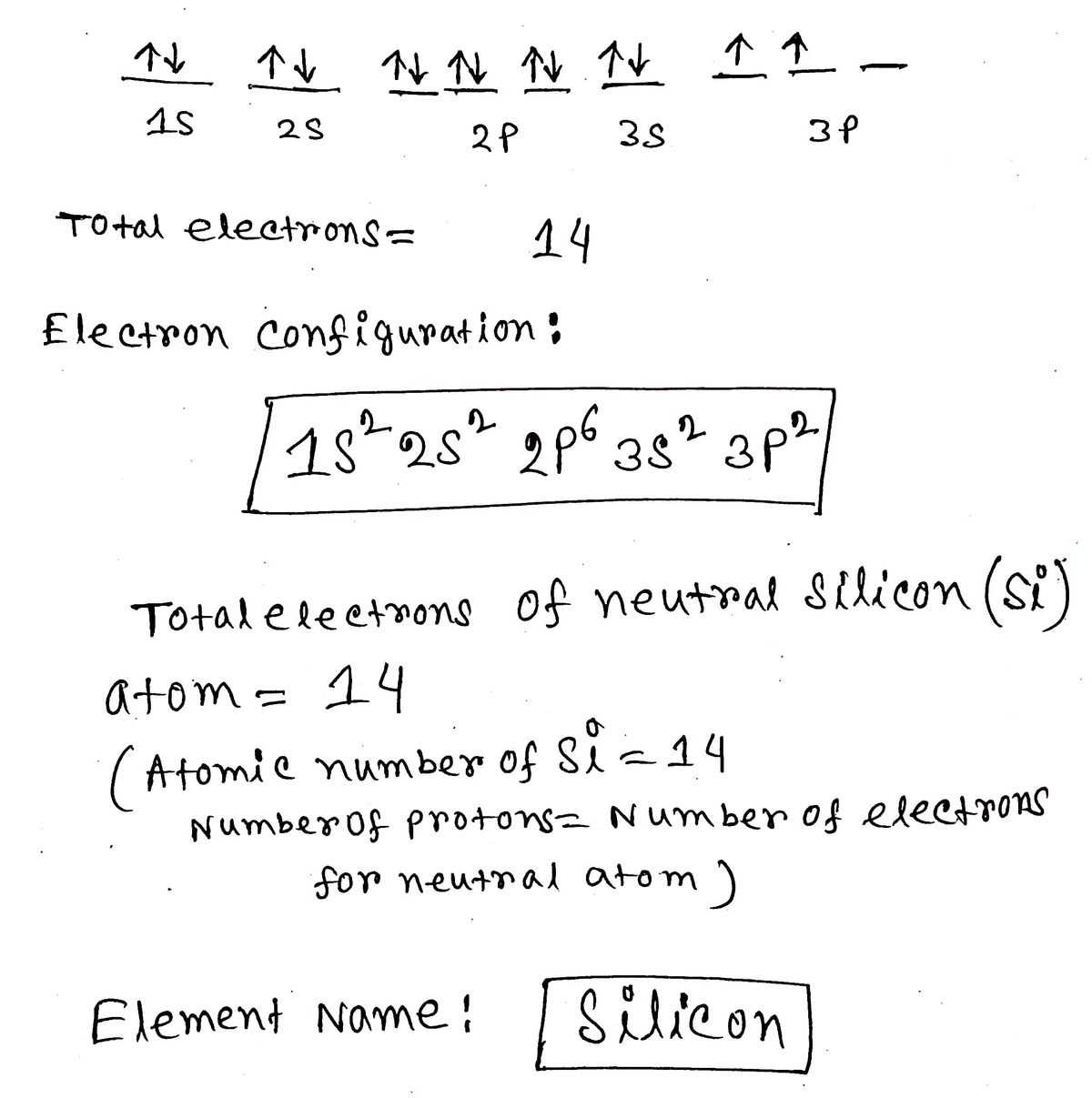
Identifying the Elements in the Configuration
Each element in the periodic table is defined by the number of protons in its nucleus, known as the atomic number. This number also determines the arrangement of electrons in the atom. By analyzing how electrons are distributed across orbitals, it is possible to determine which element is being described by a given electron arrangement. Understanding this process helps in identifying elements and predicting their chemical behavior.
The electron configuration reflects the number of electrons in an atom and their placement in different energy levels. By comparing the total number of electrons to the atomic number, it becomes clear which element is being represented. For instance, if an atom has two electrons in its first orbital and two in its second, the atomic number will point to a specific element, such as helium or beryllium.
Once the configuration is written, it can be matched with the corresponding element on the periodic table. This allows chemists to gain insights into the atom’s bonding potential, reactivity, and other properties based on its electron distribution.
Why 1s22s2 Represents Helium and Beryllium
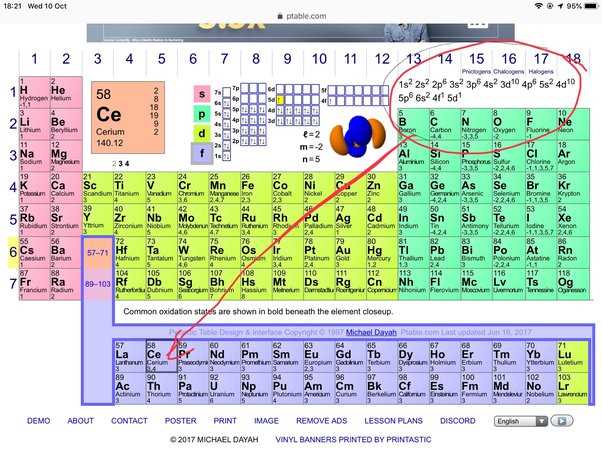
The distribution of electrons in an atom’s orbitals directly correlates with its atomic number and helps identify the element. For certain elements, like helium and beryllium, the electron configuration reflects the number of electrons present and their specific energy levels. These configurations give us a clear way to distinguish between elements based on their electron structure.
Helium: The Noble Gas with Two Electrons
Helium is a noble gas with an atomic number of 2, meaning it has two electrons. These electrons occupy the lowest energy orbital, filling the first shell. This configuration results in helium’s complete stability and is why helium is chemically inert, rarely bonding with other elements.
Beryllium: The Element with Four Electrons
Beryllium has an atomic number of 4, meaning it has four electrons. Two of these electrons fill the first shell, while the other two are placed in the second shell. This electron arrangement is characteristic of beryllium and plays a key role in its position within the periodic table and its chemical properties.
| Element | Atomic Number | Electron Configuration |
|---|---|---|
| Helium | 2 | 1s² |
| Beryllium | 4 | 1s² 2s² |
The electron configurations of helium and beryllium reflect their simple, stable structures, which are fundamental to understanding their properties and behavior in various chemical reactions.
Exploring the Concept of Energy Levels
Energy levels refer to the specific regions around an atom’s nucleus where electrons are likely to be found. These levels are integral to understanding atomic structure and the behavior of electrons. Each energy level can hold a specific number of electrons, and the further an electron is from the nucleus, the higher its energy level. The arrangement of electrons within these levels determines many of the properties of an element, including its stability and reactivity.
Structure of Energy Levels
Energy levels are often represented by numbers or shells, starting with the lowest energy level closest to the nucleus. The first energy level can hold a maximum of two electrons, while subsequent levels can accommodate more. For example, the second level holds up to eight electrons, and the third level can hold up to eighteen. As electrons fill these levels, they follow specific patterns based on the atom’s total electron count and the energy associated with each level.
Role of Energy Levels in Atomic Behavior
The arrangement of electrons within energy levels is crucial for an atom’s chemical properties. Electrons in outermost energy levels are typically the ones involved in bonding with other atoms, influencing how elements react with one another. As energy levels increase, electrons are farther from the nucleus and have higher energy, making them more likely to interact in chemical reactions.
Decoding the Notation 1s22s2
The notation used to represent the arrangement of electrons in an atom provides a shorthand for understanding an element’s electron configuration. This shorthand indicates how electrons are distributed across different orbitals, or energy levels, in the atom. Each part of the notation reveals important information about the atom’s structure, such as the number of electrons and their specific placement in various regions around the nucleus.
Understanding the Components of the Notation
In the notation, each letter and number combination has a specific meaning. The letter represents the type of orbital (s, p, d, f), and the number indicates how many electrons are in that orbital. For example, the notation “1s²” shows that two electrons occupy the first energy level in an s orbital, while “2s²” indicates that two electrons are in the second energy level in an s orbital. These details help us understand how atoms of different elements are structured and how they interact chemically.
Electron Distribution Across Orbitals
The electron configuration notation follows specific rules to reflect the most stable arrangement of electrons in an atom. Electrons fill orbitals starting from the lowest energy level, and the number of electrons per orbital is restricted based on the type of orbital. These patterns of electron distribution help explain the element’s position in the periodic table and its behavior in reactions.
| Orbital Type | Electrons in Orbital | Energy Level |
|---|---|---|
| s | 2 | 1st and 2nd |
| p | 6 | 2nd and above |
| d | 10 | 3rd and above |
This notation helps visualize the atom’s electron configuration, making it easier to understand how atoms of different elements bond, react, and form compounds.
Interpreting the Subshells and Orbitals
Atoms are structured with multiple layers or shells where electrons are arranged. These layers, also known as orbitals, are subdivided into different subshells that hold electrons in specific patterns of energy. Each subshell has a defined capacity for electrons and plays a role in determining an element’s properties, including its chemical behavior. Understanding how electrons fill these orbitals is essential for interpreting the behavior of elements in chemical reactions.
Types of Orbitals and Their Roles
Orbitals come in different types: s, p, d, and f. Each type corresponds to a different shape and energy level. The s orbitals are spherical, p orbitals are dumbbell-shaped, d orbitals have a clover-like shape, and f orbitals are even more complex. These shapes influence how electrons within these orbitals interact with other atoms, which is fundamental in determining bonding and reactivity.
Filling the Subshells
Electrons fill subshells in a specific order based on energy levels. The first subshell (1s) fills first, followed by 2s, then 2p, and so on. The rule for filling these orbitals is governed by the Aufbau principle, which states that electrons occupy the lowest available energy level first. The number of electrons each subshell can hold is determined by the orbital type: s can hold 2, p can hold 6, d can hold 10, and f can hold 14 electrons.
The Role of Quantum Numbers in Configuration
Quantum numbers play a fundamental role in determining the arrangement and behavior of electrons in an atom. These numbers provide specific information about the properties of orbitals, such as their size, shape, and orientation in space. By understanding quantum numbers, we can predict the exact location and energy of electrons, which is crucial for interpreting an atom’s electron configuration.
Types of Quantum Numbers
There are four distinct quantum numbers that describe the state of an electron within an atom:
- Principal Quantum Number (n): This number indicates the main energy level or shell of an electron. The higher the number, the greater the distance from the nucleus and the higher the energy.
- Azimuthal Quantum Number (l): This number defines the shape of the orbital. It can take integer values from 0 to n-1, where each value corresponds to a specific type of orbital (s, p, d, f).
- Magnetic Quantum Number (ml): This number specifies the orientation of the orbital within a given energy level. For each orbital type, the possible values of ml vary depending on the value of l.
- Spin Quantum Number (ms): This number describes the spin direction of the electron, either +1/2 or -1/2, representing clockwise or counterclockwise spin.
How Quantum Numbers Influence Electron Placement
The combination of these quantum numbers dictates the placement of electrons in orbitals and subshells. For instance, the principal quantum number (n) tells us which shell the electron occupies, while the azimuthal quantum number (l) determines the type of orbital (s, p, d, f) it will be in. The magnetic quantum number (ml) indicates the specific orbital within that subshell, and the spin quantum number (ms) defines the spin direction of the electron.
By understanding and applying these quantum numbers, we can fully describe the electron configuration of an atom, which in turn helps explain its chemical properties and behavior.
Why Chemical Symbols Are Important
Understanding the role of symbols in science is crucial for conveying complex information in a simple and concise manner. These notations serve as a universal language that enables scientists and students to communicate effectively across disciplines and borders. By using symbols, we can quickly describe elements, their properties, and how they interact in various reactions, making them essential for scientific progress.
Key Reasons for the Importance of Symbols
- Clarity and Efficiency: Symbols allow for the quick representation of substances and their components, avoiding the need for lengthy descriptions or full names. This efficiency is especially important in writing chemical equations and formulas.
- Standardization: The use of consistent notations ensures that scientists around the world can understand each other’s work. This uniformity is critical for collaboration and the advancement of scientific knowledge.
- Accessibility: By using symbols, information is more accessible to people across different languages and cultures, as the symbols are universally recognized.
- Understanding Reactions: These notations make it easier to interpret and predict how different substances will react with one another, based on their properties and behavior in known reactions.
The Role in Education and Research
For students and researchers, mastering these symbols is a foundational skill that helps build a deeper understanding of scientific principles. Whether it’s balancing equations or exploring molecular structures, these symbols are essential tools for engaging with the material efficiently and accurately.
How to Write Electron Configurations Properly
Writing the correct arrangement of electrons in an atom is a fundamental task in understanding atomic structure and behavior. Properly assigning electrons to specific orbitals allows for accurate predictions of chemical properties and reactivity. It is essential to follow specific rules and principles to ensure the configuration reflects the true electron distribution within an atom.
Basic Principles for Writing Electron Configurations
When assigning electrons to orbitals, there are several key principles to keep in mind:
- Aufbau Principle: Electrons fill orbitals starting from the lowest energy level, moving to higher levels as needed. This ensures the most stable electron configuration.
- Pauli Exclusion Principle: Each orbital can hold a maximum of two electrons, which must have opposite spins. This ensures no two electrons in the same atom have identical quantum numbers.
- Hund’s Rule: When electrons occupy degenerate orbitals (orbitals of the same energy), they will first fill the orbitals singly before pairing. This minimizes electron-electron repulsion and creates a more stable configuration.
Steps to Write Electron Configurations

To write the correct electron configuration, follow these steps:
- Identify the number of electrons in the atom based on its atomic number.
- Fill the orbitals in order of increasing energy levels, following the Aufbau principle.
- Apply the Pauli Exclusion Principle by ensuring no more than two electrons occupy the same orbital and that they have opposite spins.
- Use Hund’s rule when filling degenerate orbitals, ensuring that each orbital gets one electron before pairing.
By adhering to these rules, you can accurately represent the electron configuration of any element, ensuring clarity and consistency in scientific discussions.
Transition Metals and Their Electron Configurations
Transition metals occupy a unique position in the periodic table, exhibiting a range of distinct properties due to the way their electrons are arranged. These elements typically have incomplete d orbitals, and their electron configurations often deviate from simple predictions. Understanding how electrons are distributed in these metals is crucial for grasping their chemical behavior and reactivity.
Key Features of Transition Metals
- Variable Oxidation States: Transition metals can exhibit multiple oxidation states due to the ability of their electrons to occupy both the s and d orbitals. This flexibility contributes to their diverse chemical properties.
- Electron Configuration Variations: The electron configurations of transition metals often involve the filling of both s and d orbitals, and some can exhibit exceptions where electrons are moved to achieve greater stability.
- Magnetic Properties: Many transition metals show magnetic behavior, either as paramagnetic or ferromagnetic, due to the presence of unpaired electrons in their d orbitals.
Electron Configuration Patterns in Transition Metals
For most transition elements, electrons are first added to the 4s orbital before the 3d orbitals, but the exact filling order can be influenced by the need for stability. The presence of a partially filled d subshell is one of the key characteristics of these elements. The electron configuration of elements like iron or copper illustrates how electron arrangements can vary slightly to stabilize the atom.
For example, the electron configuration of copper (Cu) is [Ar] 3d10 4s1 rather than the expected [Ar] 3d9 4s2, due to the added stability of a fully filled d orbital. Such exceptions highlight the complexity of electron configurations in transition metals.
Common Trends in Transition Metal Configurations
- Elements in the same group tend to have similar electron configurations, particularly in their outermost orbitals.
- The filling of d orbitals often occurs in a way that maximizes stability, with some elements experiencing electron transfer between orbitals.
- Transition metals can have a variety of oxidation states, which arise from the ability to lose electrons from both the s and d orbitals, giving them a rich chemistry.
By examining the electron configurations of transition metals, we can predict their behavior in reactions and their ability to form complex compounds, making them essential in a wide range of industrial and chemical processes.
The Significance of 1s22s2 in Chemistry
The arrangement of electrons within an atom plays a crucial role in determining its properties and behavior. The way electrons occupy different energy levels and orbitals helps to explain the structure, reactivity, and bonding of elements. Certain configurations are particularly important, as they mark key patterns that govern the interactions between atoms. Understanding how electrons are distributed within atoms is foundational to many aspects of chemical science.
Key Concepts in Electron Configuration
- Stability and Electron Distribution: The way electrons are arranged in an atom reflects its stability. Atoms tend to achieve a configuration that minimizes energy, often following specific rules that govern electron filling.
- Bonding Behavior: The distribution of electrons influences how atoms bond with one another. Elements with similar electron configurations often exhibit similar bonding characteristics.
- Reactivity: The outermost electrons, which are the most loosely bound, largely determine an element’s reactivity. These electrons are involved in reactions with other atoms and molecules.
Importance in the Periodic Table
The electron configuration of an element is closely tied to its position in the periodic table. The arrangement of electrons determines an element’s group, period, and its tendency to form certain types of bonds. For example, elements with a fully filled outer shell are generally more stable and less reactive, while those with unpaired electrons tend to be more reactive and form stronger bonds.
- Group Trends: Elements within the same group of the periodic table often have similar electron configurations, leading to shared chemical properties and behaviors.
- Periodic Trends: The configuration of electrons across periods (horizontal rows) shows trends in atomic size, ionization energy, and electronegativity, which are fundamental to understanding the behavior of elements in reactions.
In summary, the way in which electrons are arranged and how they interact within atoms is a cornerstone of modern chemistry. It shapes not only how elements behave individually but also how they interact with others to form compounds, thus enabling the vast variety of chemical reactions that are essential to life and technology.
Electron Configuration and Periodic Table Trends
The distribution of electrons within an atom is deeply connected to its position in the periodic table. As you move across periods (rows) or down groups (columns), certain trends in atomic properties become apparent. These trends are largely a result of the way electrons are arranged in different shells and subshells. By understanding the electron arrangement, one can predict various physical and chemical properties of elements, such as atomic size, ionization energy, and electronegativity.
Trends Across Periods
As you progress from left to right across a period, the number of electrons in the outermost shell increases. This results in a greater effective nuclear charge, causing the electrons to be drawn closer to the nucleus. This trend is responsible for the decrease in atomic radius across a period. Additionally, elements in the same period generally show an increase in ionization energy and electronegativity.
- Atomic Radius: The distance between the nucleus and the outermost electron decreases across a period as the effective nuclear charge increases.
- Ionization Energy: As the atomic radius decreases, more energy is required to remove an electron from the atom.
- Electronegativity: Elements across a period tend to attract electrons more strongly due to the increased positive charge in the nucleus.
Trends Down Groups
As you move down a group, each element has an additional electron shell compared to the one above it. The increase in the number of electron shells leads to an increase in atomic size. This effect is partly counteracted by the shielding effect, where inner electrons partially block the attraction between the nucleus and the outer electrons. This results in a larger atomic radius as you move down a group, while ionization energy and electronegativity typically decrease.
- Atomic Radius: As more electron shells are added, the size of the atom increases, even though the nuclear charge also increases.
- Ionization Energy: The outermost electrons are farther from the nucleus and experience less attraction, making them easier to remove.
- Electronegativity: The ability of an atom to attract electrons decreases as the atomic radius increases, making larger atoms less electronegative.
In conclusion, the way electrons are arranged in an atom directly influences many of its fundamental properties. By examining the electron configuration, one can predict the behavior of an element in chemical reactions and understand the trends observed across the periodic table.
Common Misunderstandings in Electron Configuration
Understanding how electrons are arranged in atoms is essential for grasping many concepts in chemistry. However, there are several common misconceptions that often arise when learning about the distribution of electrons in atomic orbitals. These misunderstandings can lead to confusion when trying to predict an element’s behavior or when interpreting periodic trends. It is important to address these misconceptions to gain a clearer and more accurate understanding of atomic structure.
Misunderstanding the Order of Filling Orbitals
One common mistake is assuming that electrons always fill orbitals strictly in order of increasing energy. While the general principle is that electrons occupy the lowest energy levels first, the actual filling order is more complex. For example, the 4s orbital fills before the 3d orbital, even though the 3d orbital has a lower principal quantum number. This irregularity occurs because the energy levels of orbitals can overlap, and the relative energy of orbitals depends on factors such as shielding and electron-electron interactions.
- Misconception: Orbitals fill strictly from lowest to highest energy based on the principal quantum number.
- Correction: Some orbitals (e.g., 4s vs. 3d) fill in an unexpected order due to energy overlap.
Assuming that All Orbitals Have Equal Energy
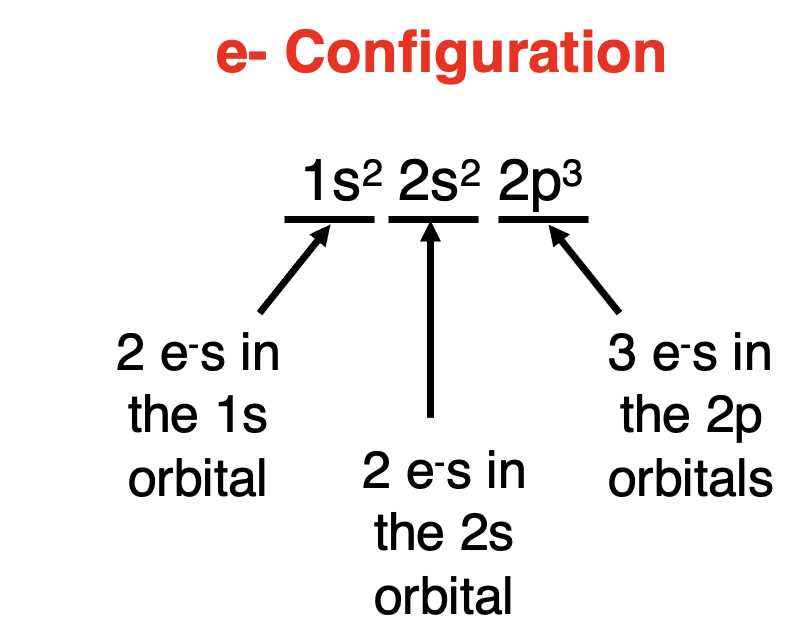
Another common misunderstanding is assuming that all orbitals within the same shell have the same energy. In reality, orbitals with different shapes (s, p, d, f) have different energy levels. For example, in the second shell, the 2s orbital is lower in energy than the three 2p orbitals. This distinction is crucial for understanding how electrons are distributed and how they influence chemical bonding and other properties.
- Misconception: All orbitals in the same shell have the same energy.
- Correction: Orbitals of different types within the same shell have different energy levels.
Neglecting the Role of Electron Pairing
Many students overlook the importance of electron pairing in orbitals. According to the Pauli Exclusion Principle, no two electrons in an atom can have the same set of quantum numbers. This means that electrons will pair up in orbitals only when necessary, which affects the electron configuration. In some cases, a higher energy level may be occupied by a single electron before pairing occurs in a lower energy orbital, especially when the atom is in an excited state.
- Misconception: Electrons will always pair up in orbitals before moving to higher energy levels.
- Correction: Electrons will occupy orbitals singly when possible, and pairing occurs only when required by the Pauli Exclusion Principle.
By understanding and correcting these common misconceptions, students can develop a deeper and more accurate understanding of electron configurations and how they relate to the properties and behavior of elements. This knowledge is essential for success in both theoretical and practical chemistry applications.