
In the field of chemistry, certain ideas serve as the foundation for understanding how substances interact, transform, and exist. These principles provide the necessary tools for scientists to quantify and explore the smallest particles that make up all forms of matter. Without a universal approach to measure these particles, it would be nearly impossible to compare substances or predict reactions with precision.
One of the key concepts involves relating microscopic particles to observable quantities, allowing chemists to work with practical numbers instead of infinitesimally small units. This system not only simplifies calculations but also bridges the gap between the atomic world and our everyday experiences. By grasping this approach, you can unlock the ability to calculate the amounts and ratios of various elements involved in chemical processes.
Understanding the Concept of the Mole
In chemistry, scientists need a way to count and quantify the tiniest particles that make up all substances. To accomplish this, they rely on a specific approach that simplifies the complex world of atoms and molecules. This concept allows for easy comparisons and calculations when working with quantities of elements and compounds in various reactions.
Rather than dealing with individual atoms, which are impractically small to measure directly, this concept provides a standard number to represent groups of particles. It essentially acts as a bridge between the invisible atomic world and the tangible quantities we can work with in laboratory settings.
To grasp the significance of this approach, it is important to understand the following points:
- It allows chemists to work with manageable amounts of particles.
- It facilitates accurate predictions of chemical reactions and reactions rates.
- It provides a common standard for various scientific disciplines to communicate and calculate values.
Ultimately, this concept is essential for anyone studying or working with chemical substances. It serves as the foundation for many calculations and plays a vital role in determining how elements and compounds interact with each other at a molecular level.
What is a Mole in Chemistry
In the field of chemistry, scientists require a system to quantify extremely small particles such as atoms and molecules in a way that is both practical and consistent. This system simplifies working with substances by using a universal standard to count vast numbers of these particles. It transforms abstract, microscopic concepts into something measurable and usable in real-world applications.
Defining the Unit

In simple terms, this standard refers to a specific number of particles, much like how a dozen refers to twelve items. The number used in this context is incredibly large, yet it allows chemists to handle quantities of atoms or molecules in ways that are both meaningful and manageable. This unit provides a foundation for calculating chemical reactions, determining atomic masses, and solving complex equations.
Practical Applications
In practical terms, using this system helps chemists to:
- Calculate the mass of substances involved in reactions.
- Predict how elements will combine to form compounds.
- Quantify the amount of a substance in terms of atoms or molecules rather than mass alone.
By using this method, chemists can work with substances in quantities that are both convenient and accurate, bridging the gap between theory and experiment. It is essential in laboratories, research, and industry, enabling precise measurements and calculations.
Significance of the Mole in Measurements
In science, accurate quantification of tiny particles is crucial for understanding how substances interact and behave. Without a reliable way to represent large quantities of atoms and molecules, it would be nearly impossible to make sense of chemical processes or predict the outcomes of reactions. This system of counting particles not only simplifies these tasks but also makes complex calculations more manageable.
By using this system, scientists can measure substances in a way that reflects their true scale, regardless of how small or large the particles involved are. This makes it possible to perform experiments, create products, and even develop new technologies based on precise quantities of elements or compounds.
Key benefits of this system in scientific measurements include:
- Allowing for consistent and reproducible results across experiments.
- Facilitating calculations in chemical equations and reactions.
- Providing a common language for chemists to communicate across different fields and industries.
In essence, this concept is vital for making sense of the atomic world, enabling scientists to work with elements and compounds in a way that is both practical and accurate. Its impact extends across all areas of chemistry, from basic research to industrial applications, and is foundational for further scientific progress.
Avogadro’s Number and Its Role
In the realm of chemistry, one of the most fundamental constants is a specific number that defines the quantity of particles in a given sample. This number allows scientists to transition from the microscopic world of atoms to the macroscopic world of substances, providing a way to work with measurable amounts of these tiny entities. It is an essential tool in bridging the gap between theoretical concepts and practical applications.
Named after the scientist who first proposed it, this constant represents the exact number of atoms, molecules, or particles in one unit of substance. It serves as the foundation for calculations involving the transformation of particles into more observable quantities, such as grams or liters.
Key aspects of Avogadro’s number include:
- It simplifies calculations by offering a clear relationship between particles and measurable amounts.
- It allows scientists to calculate the number of entities involved in chemical reactions and processes.
- It ensures consistency across different scientific disciplines when referencing atomic or molecular quantities.
By using this number, chemists can accurately predict and control reactions, determine properties of substances, and understand the behavior of materials at an atomic level. It remains an indispensable concept in all areas of chemistry, from research to industrial applications.
How Moles Relate to Atoms
Understanding the connection between the unit used to quantify substances and the atoms that make up those substances is essential in chemistry. While atoms are incredibly small and difficult to measure individually, using a specific counting system allows scientists to handle large quantities of atoms in a more practical and understandable way. This relationship is crucial for performing calculations and predictions in chemical reactions.
The Relationship Between Atoms and Units
Atoms are the basic building blocks of all matter, but working with individual atoms is not feasible in laboratory settings. Instead, scientists group these particles together using a universal counting system that represents a specific number of atoms. This connection makes it easier to calculate how atoms interact, form compounds, and behave in various chemical processes.
Why This Connection Matters
The direct link between this unit and atoms allows for the following:
- It enables scientists to calculate quantities in terms of atoms, rather than working with unmanageable numbers of individual particles.
- It provides a standard reference for comparing substances, even when their atomic structures are vastly different.
- It allows chemists to predict and understand chemical reactions on a larger scale, using a more tangible and consistent approach.
In summary, this connection provides the foundation for much of the work done in chemical analysis, helping to transform the abstract idea of atoms into practical, usable quantities for experiments, calculations, and real-world applications.
Converting Between Moles and Grams
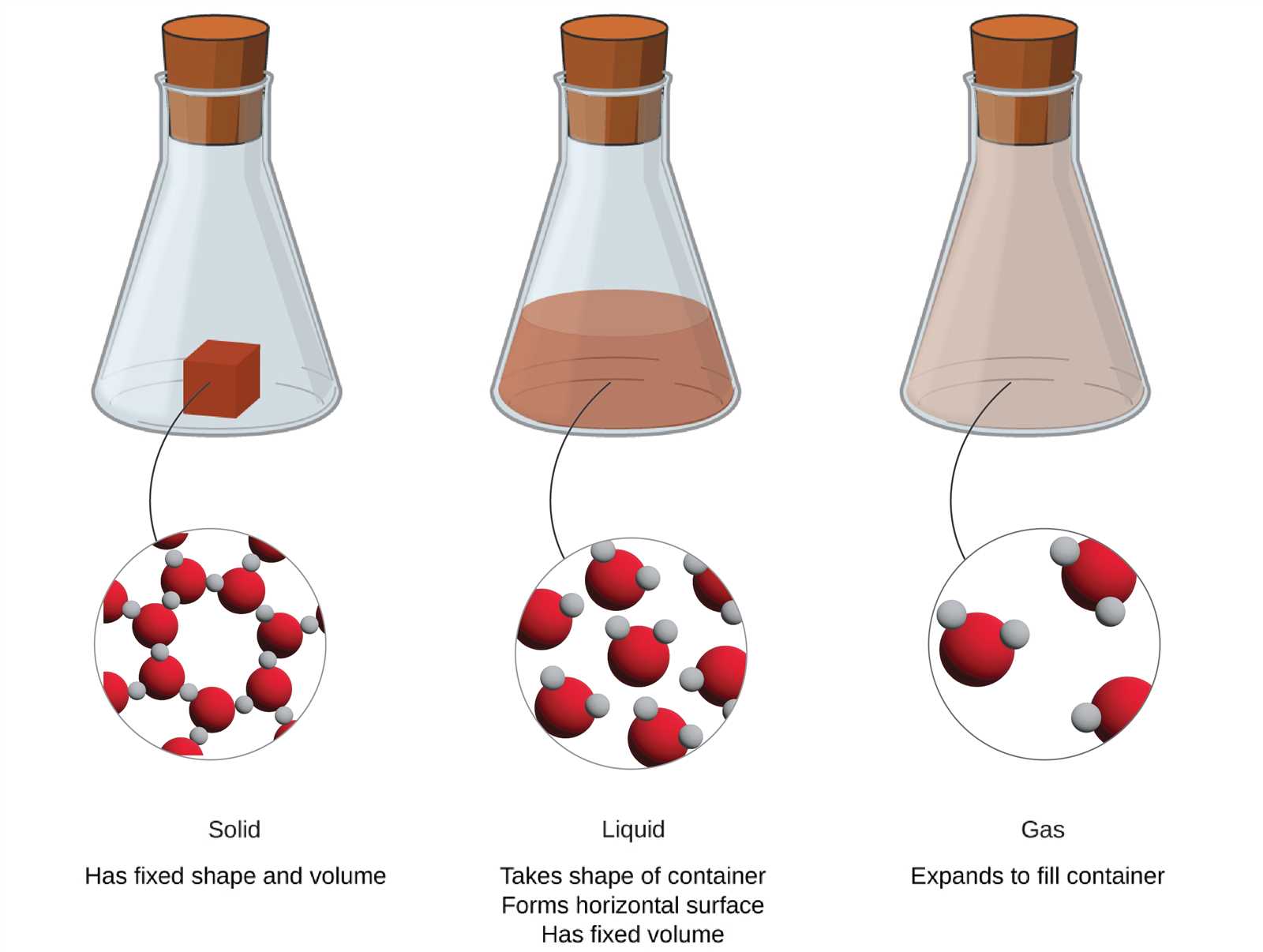
In chemistry, one of the most essential tasks is converting between different units when working with substances. Often, it is necessary to switch between the number of particles in a sample and its mass, especially when dealing with reactions and calculations. This conversion helps to relate the mass of a substance to the number of individual atoms or molecules it contains, providing a more practical way to work with quantities in the lab.
To convert between mass (in grams) and the number of particles (atoms or molecules), scientists use the relationship between the atomic or molecular mass of a substance and a specific counting unit. This process involves the use of a constant that links the mass of one unit of a substance to its number of particles, allowing for accurate conversions in both directions.
To perform a conversion, the following steps are typically used:
- Determine the molar mass of the substance, which is usually listed on the periodic table or calculated from the atomic masses of its components.
- Use the relationship between grams and moles, where one mole of any substance corresponds to its molar mass in grams.
- Apply the formula to convert from grams to moles or vice versa, depending on the required calculation.
This ability to convert between units of mass and particle count allows for easier manipulation of quantities in chemical equations, helping to ensure that experiments and reactions proceed as expected. It also serves as a bridge between different types of measurements, making it easier to calculate the required amounts of substances for any given process.
The Mole and Chemical Reactions
In chemical reactions, the ability to predict and control the quantities of substances involved is essential. Since chemical reactions occur between atoms and molecules in specific ratios, it is necessary to have a consistent way to measure and compare these quantities. By using a universal counting system, chemists can translate the microscopic interactions between particles into meaningful amounts of material that can be observed and measured in the lab.
When substances react, they do so in fixed ratios, meaning that the amount of each substance involved must be accurately calculated. This is where the relationship between particles and measurable quantities becomes crucial. The ability to convert between units of substance and countable particles allows chemists to predict how much of each reactant is needed for a complete reaction, and how much product will be produced.
In chemical equations, the coefficients represent the relative number of particles of each substance involved in the reaction. These coefficients are typically written in terms of the counting unit, allowing for easier calculations of reactant and product amounts.
| Reactant | Product | Coefficient (Particles) |
|---|---|---|
| Hydrogen (H2) | Water (H2O) | 2 : 2 |
| Oxygen (O2) | Water (H2O) | 1 : 2 |
This table illustrates a simple chemical reaction between hydrogen and oxygen, where the coefficients show the proportional amounts of each reactant and product involved. By understanding these relationships, chemists can calculate exactly how much of each substance is required to carry out a reaction and ensure that it occurs efficiently.
Practical Applications of Moles in Science
In the world of scientific research and industry, understanding and applying a specific counting system is crucial for a variety of tasks. This system allows scientists to translate theoretical concepts into real-world measurements, making it possible to calculate the quantities of substances required for experiments, products, and processes. By using this approach, researchers and engineers can accurately manipulate and measure chemical compounds in ways that are essential for progress in numerous fields.
Some of the key areas where this system plays a vital role include:
- Chemical Synthesis: In laboratories, when creating new compounds or developing pharmaceuticals, precise amounts of reactants must be used. This method ensures that chemists know exactly how much of each substance is required to achieve the desired chemical reactions.
- Environmental Science: Understanding the behavior of gases, pollutants, or natural substances in the atmosphere and water requires the ability to measure quantities of molecules accurately. This helps in tracking pollution levels, assessing environmental changes, and creating solutions to address ecological concerns.
- Pharmacology: When developing medications, it’s essential to know the correct dosage of active ingredients. This counting system allows pharmaceutical scientists to calculate and mix precise quantities of compounds for effective treatments.
- Food and Beverage Industry: In food production, the application of this counting system is used to balance ingredients, ensuring that the final product is consistent and safe for consumption. Accurate measurements are critical for maintaining quality control in large-scale production.
- Material Science: This system is vital in the development of new materials, including polymers, alloys, and nanomaterials. By understanding the atomic structure and quantity of particles, engineers can design and produce materials with specific properties for a wide range of applications.
In each of these fields, the ability to quantify and relate particles to observable quantities enables scientists and engineers to make precise predictions, perform accurate experiments, and ensure that processes run smoothly. This system is foundational to countless innovations, making it an indispensable tool in modern science and technology.
Common Mistakes in Mole Calculations
When performing calculations related to the quantity of substances involved in chemical reactions, it is easy to make errors. These mistakes can lead to inaccurate results, affecting the outcome of experiments or industrial processes. Understanding the common pitfalls in these calculations is key to avoiding them and achieving reliable results. Below are some of the most frequent errors and how to correct them.
Frequent Errors in Calculations

- Forgetting to Convert Units: One of the most common mistakes is neglecting to convert between different units. For example, failing to convert grams to moles or atoms to grams can lead to incorrect calculations and conclusions.
- Misinterpreting Molar Mass: The molar mass is often confused with atomic mass, leading to errors when converting between grams and moles. It’s important to use the correct molar mass, which is calculated based on the molecular structure, not just individual atomic masses.
- Incorrect Use of Avogadro’s Constant: Avogadro’s number is used to convert between particles and moles, but many forget to apply it correctly in calculations, resulting in faulty results.
- Not Using Proper Significant Figures: Sometimes, students round numbers too early in calculations or fail to maintain appropriate significant figures, which can significantly impact the accuracy of the final result.
- Mixing up Reactants and Products: In stoichiometric calculations, it’s crucial to correctly identify which substances are being converted. Mixing up reactants and products can lead to completely incorrect quantities.
How to Avoid These Errors
- Always Check Units: Ensure that you consistently convert between units of measurement and use the correct molar mass for the substance you are working with.
- Double Check Avogadro’s Constant: Be sure you are using Avogadro’s constant correctly when converting between particles and moles.
- Use Proper Significant Figures: Follow the rules for significant figures to ensure that your results reflect the appropriate precision.
- Carefully Identify Substances: When performing stoichiometric calculations, make sure to carefully identify reactants and products and their corresponding coefficients in the balanced chemical equation.
By being aware of these common mistakes and following these guidelines, it becomes much easier to perform accurate calculations and achieve reliable results in chemical studies and practical applications.
Units Used in Mole Measurements

In scientific work, quantifying substances accurately is crucial, and several units are used to express the amount of a substance in different contexts. These units help in translating between particles, mass, and volume, providing a universal way to understand and measure chemical quantities. Understanding these units is fundamental for conducting experiments and ensuring precise calculations in various scientific disciplines.
The most commonly used units for representing quantities of substances are:
- Grams (g): This unit is typically used to express the mass of a substance. When dealing with solid or liquid compounds, grams provide a way to quantify the amount based on weight.
- Moles (mol): The standard unit for counting quantities of atoms, molecules, or other entities. One mole represents exactly 6.022 x 1023 particles of a substance, known as Avogadro’s number.
- Particles (atoms, molecules, ions): Often used in conjunction with moles, this unit counts individual entities within a sample. It’s a way to directly refer to the number of atoms or molecules involved in reactions.
- Liters (L): Used when dealing with gases or liquids, liters measure the volume that a substance occupies. For gases, volume is often related to the number of moles through the ideal gas law.
- Atoms Per Mole (atoms/mol): This unit is used when describing the number of atoms present in one mole of a substance. It helps connect the concept of moles to individual atoms, a vital concept in stoichiometry.
Each of these units plays an important role in various scientific calculations, whether you are working with the mass of a substance, the number of particles, or the volume a substance occupies. Understanding their relationships is essential for accurate work in chemical experiments and industrial processes.
The Mole as a Bridge Between Macro and Micro
In chemistry, understanding both macroscopic and microscopic scales is essential for describing reactions, properties, and quantities of substances. At the macroscopic level, we deal with substances in bulk, measured by mass or volume, while the microscopic world involves individual particles like atoms and molecules. Connecting these two scales is critical for making sense of chemical processes, and this connection is achieved through a fundamental concept that links large quantities with the smallest entities in a substance.
This concept serves as a vital link between the visible world of substances and the invisible world of particles. Here are some key points to understand this connection:
- Linking Bulk and Particles: By using specific quantities like one mole, scientists can relate the number of particles in a substance to its measurable mass or volume. This connection allows us to handle large quantities in a way that directly correlates with molecular behavior.
- Bridging the Scale Gap: Atoms and molecules are too small to measure directly with typical tools. The concept provides a convenient way to work with them by translating microscopic quantities into macroscopic amounts that we can measure more easily.
- Predicting Chemical Reactions: The relationship between macroscopic quantities and the number of particles is crucial for understanding how substances react. Knowing how many molecules are involved in a reaction helps predict the outcome in terms of mass or volume changes in a controlled experiment.
- Scaling Chemical Calculations: Scientists use this approach to perform calculations that take into account both macroscopic measurements (like grams) and microscopic information (like molecular structure), enabling accurate predictions and measurements.
In essence, this concept provides a bridge that allows scientists to move between the everyday world we can measure directly and the invisible world of atoms and molecules. Without this key connection, we would struggle to translate observations from the laboratory into meaningful insights about how substances behave on a molecular level.
Exploring the Relationship Between Moles and Mass
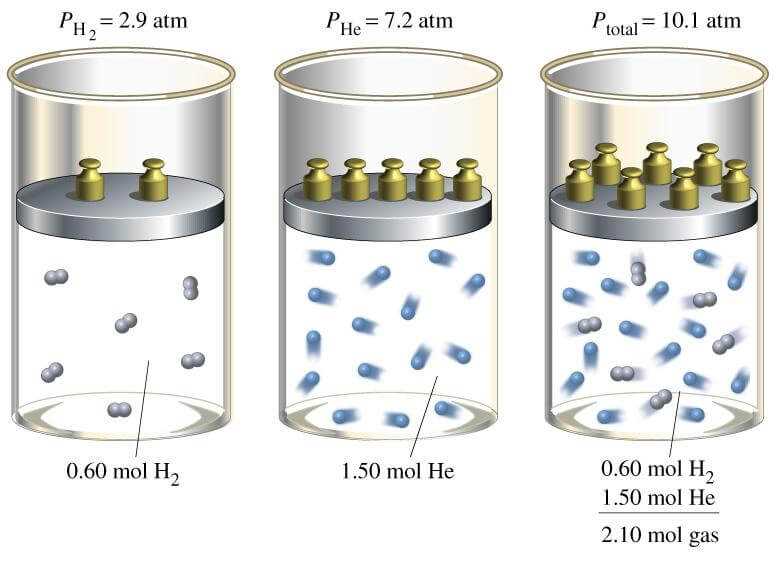
In chemical science, understanding how different quantities of substances relate to their mass is essential for performing accurate calculations and experiments. When dealing with chemical reactions, it’s vital to know how much of a substance is present, both in terms of its weight and the number of particles it contains. This connection allows chemists to predict how substances will behave during reactions and to measure them effectively.
One of the most important relationships in chemistry is how a substance’s weight, measured in grams, correlates with the amount of particles, such as atoms or molecules, it contains. This relationship makes it possible to work with macroscopic quantities while still accounting for the behavior of individual molecules at the microscopic level. Here’s how the two are related:
- Mass and Particle Number: The mass of a substance is directly related to the number of particles it contains. By knowing the molecular weight of a substance, chemists can calculate how many particles are present in a given mass.
- Atomic Mass and Molecular Weight: The atomic mass of an element or the molecular weight of a compound is a key factor in this relationship. It allows scientists to determine how much one mole of a substance weighs, which in turn enables the calculation of how many moles are in a specific mass of a substance.
- Calculating Mass from Moles: By using the molecular weight, you can easily convert moles of a substance into its corresponding mass. This is crucial for accurately measuring and mixing chemicals in the correct proportions for experiments.
- Stoichiometry and Reaction Predictions: The relationship between mass and moles allows chemists to predict the outcome of chemical reactions. By knowing the mass of reactants and products, it’s possible to determine how much of each will be involved in the reaction.
This relationship is fundamental to many calculations in chemistry, particularly when working with reactions, molar ratios, and when precise measurements are needed for experimental design. Understanding the connection between the number of particles and the mass they represent is a cornerstone of chemical theory and practice.
How to Calculate Moles from Mass
To accurately determine the number of particles present in a given sample, scientists often need to calculate how many “units” are contained within a specific mass of substance. The process involves converting the given mass into moles, a fundamental concept in chemistry. By doing so, it’s possible to bridge the gap between macroscopic measurements and microscopic entities like atoms or molecules.
Steps for Calculation
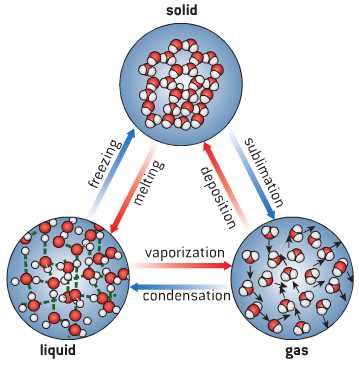
To calculate moles from mass, follow these straightforward steps:
- Obtain the Mass: Start by determining the mass of the substance you’re working with. This is usually measured in grams.
- Find the Molecular Weight: Next, look up the molecular weight (or atomic mass for elements) of the substance. This is typically listed on the periodic table and represents the mass of one mole of that substance, measured in grams per mole (g/mol).
- Use the Formula: The basic formula for converting mass to moles is:
Moles = Mass (grams) / Molar Mass (g/mol)
Example Calculation
Suppose you have 20 grams of a substance, and its molar mass is 40 g/mol. To find the number of moles:
Moles = 20 g / 40 g/mol = 0.5 moles
In this example, you have 0.5 moles of the substance in the sample. This method can be applied to any chemical substance as long as you know its mass and molar mass.
By using this calculation, chemists can more easily work with the quantities of substances in their experiments, ensuring accuracy and consistency in their work.
Understanding Molar Volume in Gases
In chemistry, it is essential to understand how gases behave under different conditions, particularly when considering their volume. One of the key concepts in this area is the volume occupied by a specific amount of gas particles, often referred to as the “molar volume.” This concept allows scientists to link the macroscopic properties of gases, such as their volume, to the number of particles present.
What is Molar Volume?
Molar volume refers to the volume that one mole of a gas occupies at standard conditions. These standard conditions are typically defined as a temperature of 0°C (273.15 K) and a pressure of 1 atmosphere (atm). At these conditions, one mole of any ideal gas will occupy a volume of approximately 22.4 liters. This value is known as the standard molar volume of an ideal gas.
Factors Affecting Molar Volume
While the standard molar volume for gases is often quoted as 22.4 L/mol, it is important to recognize that real gases may deviate slightly from this value due to factors such as:
- Temperature: As the temperature of a gas increases, its particles move faster and may expand, increasing the volume.
- Pressure: High pressure can compress gas particles, reducing the volume they occupy.
- Gas Type: Ideal gases follow this rule, but real gases may show slight variations based on intermolecular forces.
Understanding the molar volume of gases provides a crucial link between the microscopic world of particles and the macroscopic properties we can observe and measure. This principle is fundamental in gas laws and various chemical calculations, making it a vital concept in both theoretical and applied chemistry.
The Mole in Stoichiometry Calculations
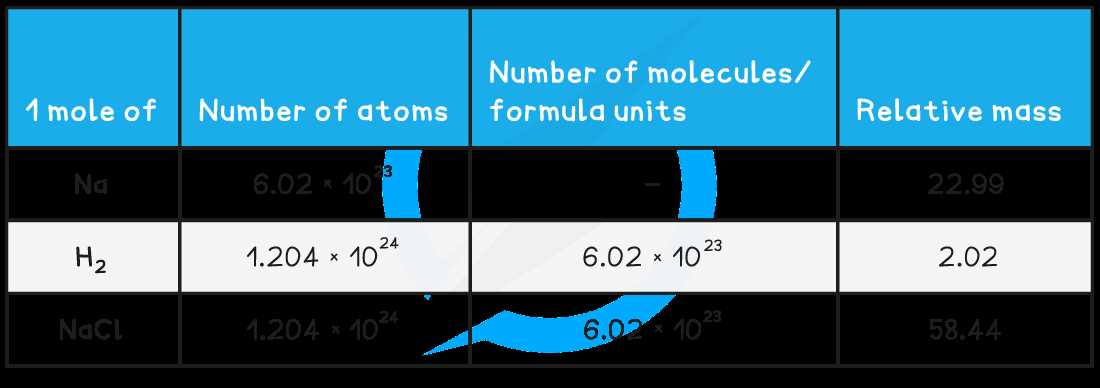
In chemical reactions, understanding the relationship between different substances is essential for making accurate calculations. Stoichiometry, the study of the quantitative relationships between reactants and products in a chemical reaction, relies heavily on converting between different units such as mass, volume, and particles. The key to these conversions is a specific quantity that allows chemists to relate microscopic particles to measurable amounts.
Using Moles in Stoichiometric Ratios
In stoichiometry, the amount of a substance is often expressed in terms of moles. By using the balanced chemical equation for a reaction, chemists can determine the ratio of reactants to products. This ratio, when combined with the amount of one substance, allows the calculation of the amounts of other substances involved in the reaction.
For example, in a reaction between two reactants, knowing the moles of one reactant can help determine the moles of the second reactant or the product. This relationship is essential for predicting how much of a product will be formed or how much of a reactant is required for a given amount of product.
Step-by-Step Stoichiometric Calculations
When performing stoichiometric calculations, several steps are involved to ensure accuracy:
- Balance the chemical equation: Ensure that the number of atoms on both sides of the equation is equal.
- Convert known quantities to moles: Use the molar mass of a substance to convert between grams and moles.
- Apply the stoichiometric ratio: Use the coefficients from the balanced equation to set up a conversion factor between substances.
- Calculate the unknown quantity: Once the appropriate ratio is established, calculate the required or produced amount.
By mastering these calculations, chemists can predict the outcomes of chemical reactions with precision, making stoichiometry a fundamental tool in both laboratory and industrial chemistry.
Real-Life Examples of Mole Usage
The concept of quantifying substances is vital in everyday life, especially in industries where precise chemical reactions are required. The ability to measure and calculate substances in consistent units enables us to produce products, conduct experiments, and solve real-world problems. In various fields, from pharmaceuticals to environmental science, understanding how to convert between small and large quantities of matter is fundamental for achieving accuracy and efficiency.
Pharmaceutical Industry
In drug formulation, precise amounts of chemical compounds are necessary to ensure effectiveness and safety. When preparing medications, chemists must calculate how much of each substance is needed to create a dose. The use of specific quantities, often measured in moles, ensures that the active ingredients in a pill or liquid medication are correctly proportioned.
Environmental Science and Pollution Control
In environmental chemistry, understanding the composition of pollutants is critical. For instance, determining the amount of a harmful substance in the air, water, or soil often requires converting from moles to measurable quantities such as parts per million (ppm). This conversion allows scientists to assess the environmental impact and take corrective actions, such as cleaning up contaminated sites.
Table of Real-Life Mole Usage Examples
| Industry | Application | Substance Involved | Why Moles Are Used |
|---|---|---|---|
| Pharmaceuticals | Drug dosage preparation | Active ingredients | To ensure accurate concentration of substances |
| Environmental Science | Pollution monitoring | Contaminants in air or water | For determining safe limits and pollutant removal |
| Chemical Manufacturing | Production of fertilizers | Nitrogen, phosphorus, potassium | To mix precise ratios for optimal plant growth |
| Food Industry | Food preservation and additives | Preservatives, acids, flavoring agents | To maintain consistency and safety in products |
These examples highlight the essential role of this quantity in various fields. Whether it’s ensuring the correct amount of a substance is used in drug production or analyzing pollutant levels in nature, understanding how to work with moles facilitates the proper application of science in real-world scenarios.
Challenges in Teaching the Concept of Moles
Teaching the fundamental idea of quantifying substances in chemistry can present several challenges. Understanding how to relate small-scale particles to larger, measurable amounts requires students to bridge abstract concepts with practical applications. Many students find it difficult to grasp the relationship between individual atoms or molecules and bulk quantities, as well as to visualize these particles on a scale they can’t directly observe. Teachers often face the task of making this concept both accessible and meaningful.
Common Struggles Faced by Students
Students often encounter several difficulties when learning how to apply this concept in calculations and real-world situations. These struggles stem from both the abstract nature of the idea and the complexity of the mathematical formulas involved. Some of the most common challenges include:
- Difficulty in understanding the scale of particles versus macroscopic quantities
- Confusion between different units and conversion factors
- Challenges in applying concepts to stoichiometry and other chemical equations
- Difficulty in visualizing how substances interact on a molecular level
Teaching Strategies to Overcome These Challenges
To address these struggles, teachers can adopt various strategies to help students build a better understanding of the topic. Incorporating visual aids, hands-on activities, and real-life examples can help students connect abstract ideas with tangible experiences. Here are some effective approaches:
- Using visual models to represent atoms, molecules, and bulk substances
- Breaking down complex calculations into smaller, manageable steps
- Providing interactive exercises that allow students to practice converting between different units
- Introducing real-world scenarios to show the relevance of the concept in various industries
Table of Common Challenges and Solutions
| Challenge | Solution |
|---|---|
| Difficulty in visualizing the scale of particles | Use of models, animations, or simulations to illustrate particle behavior |
| Confusion with unit conversions | Step-by-step breakdown of unit conversion techniques and practice problems |
| Struggles with stoichiometry and chemical equations | Use of guided practice and interactive exercises to reinforce key concepts |
| Understanding the practical applications | Incorporation of real-life examples from industries such as pharmaceuticals, environmental science, and food production |
By addressing these common struggles and applying these strategies, educators can help students better understand and apply the concept of quantifying substances. This understanding is crucial not only for academic success but also for real-world applications in various scientific fields.