
Preparing for significant assessments requires a thorough understanding of core principles and a well-structured strategy. Whether it’s recalling key concepts or solving complex problems, having a clear approach is essential for achieving excellent results.
This guide is designed to assist learners in navigating the most important topics, offering clarity on challenging subjects and practical methods to enhance retention. By focusing on structured practice and targeted learning, it aims to boost confidence and understanding in essential areas.
Mastering core ideas and strengthening problem-solving skills are crucial steps toward success. By engaging with carefully curated resources and exercises, students can refine their knowledge and address any gaps effectively. Embrace this opportunity to enhance your academic journey.
Effective Study Techniques for Test Preparation
Getting ready for an important test requires a balanced approach that combines understanding concepts, practicing application, and managing time efficiently. A well-organized plan ensures better retention and a clear grasp of the material.
- Create a structured schedule: Allocate specific times for studying different topics to cover all areas thoroughly.
- Focus on fundamental principles: Build a solid foundation by revisiting the basic ideas that support more advanced topics.
- Use visual aids: Diagrams, charts, and tables can help simplify complex ideas and make them easier to recall.
Regular practice is essential to strengthen problem-solving abilities and enhance confidence. Testing your knowledge with mock quizzes and sample exercises can reveal weak areas that need further attention.
- Start with topics you find most challenging to ensure maximum focus.
- Work through practice problems step-by-step to understand the logic behind each solution.
- Review errors carefully to avoid repeating them in the actual test.
Finally, maintaining a healthy balance between study sessions and rest is crucial for staying motivated and avoiding burnout. Effective preparation is not just about hard work but also smart strategies that lead to better outcomes.
Key Concepts to Master in Chemistry
Understanding the foundational principles of science is essential for building a deeper comprehension of more advanced topics. Focusing on these areas provides a strong framework for tackling complex questions and solving practical problems effectively.
Fundamental Ideas to Focus On

Core topics include the study of matter, interactions between substances, and the rules governing these changes. Mastering these ideas helps in analyzing real-world applications and theoretical scenarios alike.
| Concept | Importance | Application |
|---|---|---|
| Structure of substances | Explains the behavior of materials | Designing new materials |
| Energy changes | Key to understanding reactions | Optimizing industrial processes |
| Quantitative relationships | Ensures accurate calculations | Laboratory experiments |
Practical Strategies for Success

To fully grasp these principles, practice solving problems related to these topics and review examples that illustrate their applications. Using visual aids, such as diagrams and graphs, can further simplify complex ideas and reinforce understanding.
How to Solve Chemical Equations Easily

Balancing reactions is a fundamental skill that requires a clear understanding of the relationships between reactants and products. Mastering this process ensures accuracy in predicting outcomes and measuring quantities.
Begin by identifying all substances involved in the reaction. Ensure that the number of each type of atom is the same on both sides of the equation. This principle reflects the idea that matter is conserved and cannot be created or destroyed.
Use coefficients to adjust the quantities of compounds or elements. Focus on balancing one type of atom at a time, starting with those that appear in the fewest substances. Leave hydrogen and oxygen for last, as they are often more complex.
To simplify the process:
- Write out all components clearly to avoid confusion.
- Double-check your work to confirm balance and consistency.
- Practice with examples of varying difficulty to build confidence.
Regular practice and familiarity with different reaction types will make balancing equations more intuitive and efficient over time.
Strategies for Memorizing Periodic Table Trends
Understanding the patterns within the table of elements is essential for predicting their properties and behaviors. Recognizing these trends simplifies problem-solving and aids in connecting theoretical knowledge to practical applications.
To effectively retain these patterns, start by grouping elements based on their shared characteristics. Focus on how properties such as size, reactivity, and energy levels change across rows and down columns. Associating these trends with visual cues or mnemonic devices can make the process more intuitive.
Key approaches include:
- Using color-coded diagrams: Highlight changes in properties like metallic character or electronegativity to identify relationships visually.
- Creating memory aids: Develop simple phrases or acronyms to recall sequences and changes.
- Practicing with examples: Apply these patterns to real-world scenarios or exercises to solidify understanding.
By breaking down the table into manageable sections and repeatedly engaging with its layout, learners can improve their recall and confidence in utilizing this valuable tool.
Understanding Molecular Structures and Bonds
The arrangement of atoms and the forces that hold them together play a crucial role in determining the properties and behavior of substances. Grasping these concepts allows for a deeper understanding of how interactions occur on a fundamental level.
Key Features of Molecular Structures
To comprehend how substances are formed and interact, it is important to focus on the shapes, angles, and arrangements of their components. These features dictate properties like polarity, reactivity, and physical states.
- Geometry: Recognize common shapes such as linear, bent, or tetrahedral, which influence interactions between particles.
- Electron distribution: Understand how shared or unshared electron pairs impact the stability and structure.
- Hybridization: Learn how atomic orbitals combine to form unique bonding arrangements.
Types of Interactions
The connections between particles can vary in strength and nature. Recognizing these differences helps predict behavior in reactions and under various conditions.
- Strong forces: Includes ionic and covalent interactions that form the framework of substances.
- Weaker interactions: Such as hydrogen or Van der Waals forces, which influence properties like boiling points.
- Delocalization: Occurs when electrons are shared across multiple centers, contributing to unique characteristics.
By analyzing structures and their connections, learners can build a comprehensive understanding of how materials function and respond to external influences.
Practice Problems for Organic Chemistry Topics

Engaging with practice exercises is essential for mastering complex concepts in the study of carbon-based compounds. These exercises not only reinforce theoretical knowledge but also help build problem-solving skills necessary for deeper understanding.
Start by focusing on basic concepts such as functional groups, naming conventions, and reaction mechanisms. As you progress, challenge yourself with problems that require applying these ideas to real-world scenarios or unfamiliar compounds.
Below are a few practice problems designed to sharpen your understanding:
- Identify the functional group: Given a series of molecules, determine the functional group present in each structure (e.g., alcohol, aldehyde, carboxylic acid).
- Reaction mechanisms: For a given reaction, outline the steps involved in the transformation of reactants to products, showing electron movement.
- Isomerism: Identify and distinguish between structural isomers and stereoisomers in a set of organic compounds.
- Naming compounds: Practice IUPAC nomenclature by naming a series of organic compounds based on their structures.
These exercises will help you develop the necessary skills to analyze and synthesize compounds while preparing for more advanced topics.
Tips for Tackling Chemistry Calculations
Solving numerical problems is a vital skill in understanding the practical aspects of substances and reactions. Mastering calculations enhances your ability to apply theoretical concepts to real-world situations, allowing you to predict behaviors, quantities, and outcomes with precision.
When faced with calculations, start by ensuring you fully understand the problem. Identify the known values and what is being asked, then organize your approach step by step. It’s important to focus on unit conversions, balancing equations, and correctly applying formulas to reach the correct result.
- Identify the right formula: Make sure you choose the correct equation based on the given information. Common formulas include those for molarity, stoichiometry, and gas laws.
- Convert units carefully: Pay attention to unit conversions, as they are crucial for accurate results. Always check if units need to be converted before applying them in calculations.
- Balance equations: Before using stoichiometric ratios, ensure the equation is balanced. This is fundamental for solving problems related to reactants and products.
- Show all steps: Writing out each step helps ensure you don’t miss any important calculations and makes it easier to spot errors in your work.
By developing a methodical approach to calculations, you can minimize mistakes and gain confidence in solving more complex problems as you advance.
Common Mistakes in Chemistry Exams

While preparing for assessments in scientific subjects, it is essential to be aware of the most frequent errors students make. These mistakes can often be avoided with careful attention to detail and a systematic approach to problem-solving.
One common issue is misinterpreting the question. It’s easy to rush through reading the problem and miss critical details, leading to incorrect answers. Another frequent mistake is neglecting unit conversions or failing to convert between different forms, which can result in inaccurate calculations.
- Forgetting to balance equations: Before applying stoichiometric ratios, ensure that all chemical equations are properly balanced to avoid errors in the calculations.
- Misunderstanding the concept of limiting reactants: Often, students focus too much on one reactant and fail to identify the limiting reactant, leading to incorrect results.
- Overlooking significant figures: It’s important to account for significant figures in calculations to ensure precision and avoid losing accuracy in the final answer.
- Rushing through calculations: Skipping steps or performing calculations too quickly can lead to simple arithmetic mistakes that could affect the outcome of the problem.
By recognizing and addressing these common mistakes, students can improve their performance and avoid unnecessary errors during their assessments.
Effective Study Habits for Chemistry Success
Mastering complex scientific topics requires a combination of strategic study habits and consistent effort. Building a solid foundation is key, but it’s also crucial to develop efficient methods that promote long-term retention and understanding.
One of the most effective strategies is breaking down material into manageable sections. Rather than trying to learn everything in one sitting, focus on small chunks of information and reinforce your understanding through regular review sessions. Another useful approach is active recall, where you actively test yourself on the material rather than passively reading through notes.
- Create a study schedule: Establish a routine that allocates specific times for studying, helping to maintain focus and consistency.
- Practice regularly: Work through problems and exercises to apply concepts and strengthen your problem-solving skills.
- Stay organized: Keep notes, formulas, and resources organized for easy access, which will help you quickly find the information you need during study sessions.
- Use study groups: Discussing topics with classmates can help clarify complex concepts and provide different perspectives on challenging material.
By incorporating these habits into your study routine, you’ll be able to enhance your grasp of key concepts and perform with confidence in assessments.
Time Management During Chemistry Tests
Effectively managing time during a scientific assessment is essential for success. Proper planning and pacing can ensure that each question receives the attention it requires without running out of time. A well-organized approach helps minimize stress and maximize performance.
One key strategy is to quickly assess the length and difficulty of the test. Start by scanning the entire paper to understand how much time to allocate to each section. Identify the questions that seem easier or quicker to solve and tackle them first, leaving more complex problems for later. This approach allows for building momentum and reduces anxiety.
- Prioritize your time: Spend less time on questions you find easy and more time on challenging ones. Balance is crucial to avoid getting stuck on any single item.
- Set time limits: For each section or question, set a reasonable time limit and stick to it, ensuring that you stay on track.
- Leave time for review: Set aside the last few minutes to go back over your work, checking for errors or incomplete answers.
- Stay calm and focused: If you find yourself running out of time, don’t panic. Focus on completing as many questions as possible with the remaining time.
By managing your time effectively, you can reduce stress and improve your ability to answer questions thoroughly within the given timeframe.
Best Resources for Chemistry Exam Review
When preparing for a scientific assessment, having access to quality resources can make all the difference. With the right materials, you can reinforce your understanding, clarify difficult concepts, and practice solving problems. Whether you’re looking for textbooks, online platforms, or study groups, the resources you choose can greatly enhance your ability to perform well.
Online Platforms and Websites
One of the most convenient ways to prepare is by using online resources. These platforms often provide interactive tutorials, practice questions, and video lessons on a wide range of topics. Here are some great options:
- Khan Academy: Offers free, in-depth lessons on a variety of scientific topics, including interactive exercises and quizzes.
- Coursera: Provides online courses from universities that cover foundational concepts in the subject, often with peer-reviewed assignments.
- Quizlet: A useful platform for finding flashcards and practice tests created by other students or educators.
Study Guides and Textbooks

Another reliable resource for mastering scientific concepts is a good textbook or study guide. These often break down complex material into more digestible sections and provide practice problems with detailed solutions. Some popular choices include:
- Organic Chemistry As a Second Language: A great study guide focused on organic concepts, offering simple explanations and plenty of examples.
- Mastering Chemistry (Pearson): A comprehensive textbook with integrated learning tools, such as practice problems and visual aids.
- McGraw-Hill’s Chemistry Study Guide: A well-organized study aid with concise explanations and useful strategies for solving problems.
These resources can help you strengthen your foundation, practice problem-solving, and approach the material from multiple angles, improving your chances of success.
Preparing for Multiple-Choice Questions
Approaching multiple-choice questions with a strategic mindset is crucial for achieving the best results. These types of questions often test both knowledge and the ability to apply concepts in different contexts. By mastering specific strategies, you can effectively navigate through the options and identify the correct choice with confidence.
One of the first steps is to thoroughly understand the core concepts that are being tested. This allows you to recognize the most likely correct answers. Another essential technique is to carefully read each question and all the options before selecting an answer, as sometimes a subtle detail can make a significant difference. Additionally, it’s important to rule out obviously incorrect choices and focus on the remaining options to improve your chances of making the right decision.
Here are some practical tips to enhance your preparation for multiple-choice questions:
- Focus on Key Concepts: Understand the main ideas rather than just memorizing facts. This helps in eliminating incorrect answers more easily.
- Eliminate Wrong Answers: Often, two or more options will be clearly incorrect, so narrowing down your choices increases the likelihood of choosing the right one.
- Don’t Overthink: Trust your first instinct. Overanalyzing can sometimes lead to confusion, so rely on your initial judgment if you’re unsure.
- Practice Regularly: Taking practice tests can simulate the real scenario and help you develop a faster, more effective approach to answering these questions.
By using these techniques, you can approach multiple-choice questions with a clearer mindset, improving your performance and confidence in answering them accurately. Practice and preparation are key to mastering this type of questioning format.
Improving Skills in Laboratory Techniques
Mastering laboratory techniques is essential for conducting accurate and effective experiments. Whether you’re preparing solutions, measuring substances, or observing reactions, the precision and efficiency with which you perform these tasks can significantly impact the results. Building these skills requires a combination of proper practice, understanding of equipment, and awareness of safety protocols.
One of the most important aspects of improving laboratory skills is developing a strong familiarity with the tools and instruments used in experiments. From burettes to pipettes, understanding how each piece of equipment works and when to use it will help you achieve more precise measurements. Additionally, following step-by-step procedures carefully ensures that the experiment proceeds smoothly, reducing the chances of errors.
Another key factor is maintaining a high standard of organization. Keeping your workspace clean and properly arranged allows for easier access to materials and minimizes the risk of contamination. Furthermore, being mindful of time management can improve efficiency, ensuring that experiments are completed within the required time frame without sacrificing quality.
- Practice Proper Technique: Familiarize yourself with the correct methods for measuring, mixing, and handling substances. Consistent practice enhances precision.
- Understand the Equipment: Learn the functions and correct usage of laboratory instruments to avoid mistakes during experiments.
- Prioritize Safety: Always follow safety guidelines to prevent accidents and protect both yourself and others in the lab.
- Organize Your Workspace: A clean, orderly workspace improves efficiency and reduces the likelihood of errors.
Regular practice, careful attention to detail, and a commitment to following best practices are all necessary to refine laboratory skills. With these strategies, you can ensure more reliable outcomes and foster a deeper understanding of the processes involved in scientific experimentation.
Analyzing Graphs and Data in Science
Interpreting data and graphs plays a crucial role in understanding experimental results and drawing conclusions. By analyzing visual representations of data, you can identify patterns, trends, and relationships between variables. This skill is essential for making informed decisions and evaluating the success of a particular experiment or investigation.
Graphs are often used to simplify complex data, allowing for quicker understanding. For example, a line graph can show how one variable changes in response to another, making it easier to identify correlations. Bar graphs, on the other hand, are useful for comparing quantities across different categories. Proper analysis of these graphs involves looking for trends, calculating slopes, and recognizing any anomalies in the data.
- Understand the Variables: Identify the independent and dependent variables in the graph. This helps to understand what is being measured and how the two are related.
- Examine the Scale: Pay attention to the scale of both axes. Uneven scales or misleading axes can distort the data interpretation.
- Look for Patterns: Identify trends, such as increasing or decreasing patterns, that can help explain the relationship between variables.
- Analyze Anomalies: Look for outliers or unusual data points. These may indicate errors or unexpected results that require further investigation.
- Use Slope for Rate of Change: In graphs showing continuous data, the slope can represent the rate of change between variables, which can be useful for understanding underlying trends.
When dealing with complex datasets, it’s also helpful to organize the data first, ensuring that it is accurate and consistent before graphing. Additionally, drawing conclusions based on graphical data should be done cautiously, as visual trends may not always represent the full scope of the data. It’s essential to cross-reference with raw numbers and calculations to support findings.
Reviewing Key Vocabulary in Science
Understanding essential terminology is fundamental for mastering scientific concepts. It allows you to communicate ideas effectively and accurately interpret complex ideas and processes. A solid grasp of core terms will also help you better understand theoretical concepts, laboratory procedures, and problem-solving techniques, which are central to any scientific discipline.
Common Terms and Definitions

Below is a table of frequently used terms in the subject, along with their corresponding definitions to help reinforce their meanings:
| Term | Definition |
|---|---|
| Atom | The smallest unit of matter, consisting of a nucleus and electrons. |
| Molecule | A group of atoms bonded together, representing the smallest fundamental unit of a compound. |
| Covalent Bond | A chemical bond formed by the sharing of electrons between atoms. |
| Ionic Bond | A type of chemical bond formed by the transfer of electrons from one atom to another. |
| Reaction Rate | The speed at which a chemical reaction occurs, often affected by concentration, temperature, and catalysts. |
| Electronegativity | A measure of the ability of an atom to attract shared electrons in a chemical bond. |
Tips for Memorizing Vocabulary

- Use Flashcards: Create flashcards with the term on one side and the definition on the other to test your recall.
- Relate Terms to Real-World Examples: Find real-life examples where the term is applied to reinforce its meaning.
- Group Related Terms: Organize terms into categories (e.g., bonding types, atomic structure) to see their connections.
- Practice Writing Definitions: Rewriting definitions in your own words can help cement the term’s meaning.
- Quiz Yourself Regularly: Self-testing helps retain vocabulary and improves recognition during assessments.
Understanding Acids, Bases, and pH Levels
Acids and bases are fundamental to understanding various processes in the natural world, including those in living organisms and industrial applications. These substances interact with each other in specific ways, often resulting in neutralization reactions. The strength of acids and bases is measured on a scale known as pH, which determines whether a solution is acidic, neutral, or basic. Understanding this balance is crucial for fields ranging from biology to environmental science.
Acids are substances that release hydrogen ions (H+) when dissolved in water, while bases release hydroxide ions (OH-). A solution’s pH level indicates the concentration of hydrogen ions present. A pH below 7 indicates an acidic solution, a pH of 7 signifies a neutral solution, and a pH above 7 points to a basic (alkaline) solution.
Here are some key concepts to consider when studying the behavior of acids and bases:
- Strength of Acids and Bases: The strength of an acid or base refers to its ability to dissociate in water. Strong acids and bases dissociate completely, while weak acids and bases only partially dissociate.
- Neutralization Reactions: When an acid and a base are mixed, they react to form water and a salt, effectively neutralizing each other.
- Buffer Solutions: Buffers are substances that help maintain a stable pH in a solution, preventing drastic changes in acidity or alkalinity.
- pH Scale: The pH scale ranges from 0 to 14, where values below 7 are acidic, values above 7 are basic, and a value of 7 is neutral.
Being able to measure and understand pH levels is vital in various applications, such as in water treatment, agricultural practices, and medical diagnostics. A strong foundation in the behavior of acids, bases, and their pH levels is essential for success in these fields.
Mastering Stoichiometry for Better Exam Results
Mastering the art of balancing equations and calculating the relationships between reactants and products is a critical skill for achieving success in problem-solving. Stoichiometry involves the precise calculation of the quantities of substances involved in chemical reactions. By becoming proficient in these calculations, students can improve their accuracy and confidence when tackling complex problems.
Key Concepts to Focus On
Understanding the following concepts is essential for mastering stoichiometry:
- Balanced Equations: Before diving into calculations, ensure that the chemical equation is balanced. This ensures that the law of conservation of mass is satisfied and that the ratio of reactants to products is correct.
- Molar Ratios: The coefficients in a balanced chemical equation represent the molar ratios of reactants to products. These ratios are crucial when converting between moles of different substances.
- Dimensional Analysis: Use dimensional analysis to convert between units such as grams, moles, and liters. This technique helps in ensuring that all units cancel out properly, leading to the correct answer.
Practical Steps for Solving Stoichiometry Problems

Follow these steps to solve stoichiometric problems effectively:
- Start with the known quantity, usually given in the problem statement.
- Convert this quantity into moles if necessary.
- Use the mole ratios from the balanced equation to relate the quantities of different substances.
- Convert the resulting moles into the desired unit (grams, liters, molecules, etc.).
By practicing these steps regularly, students can gain a deeper understanding of how substances interact in reactions and improve their problem-solving efficiency. Mastery of stoichiometry is a valuable tool for achieving strong results in any assessment involving chemical calculations.
Review Checklist for Chemistry Exams

Preparing for a test that involves complex concepts requires a strategic approach. Having a well-organized checklist can help ensure all critical topics are covered thoroughly. This guide provides a structured approach to reviewing key areas that are essential for performing well in assessments related to chemical principles and problem-solving.
Essential Topics to Review
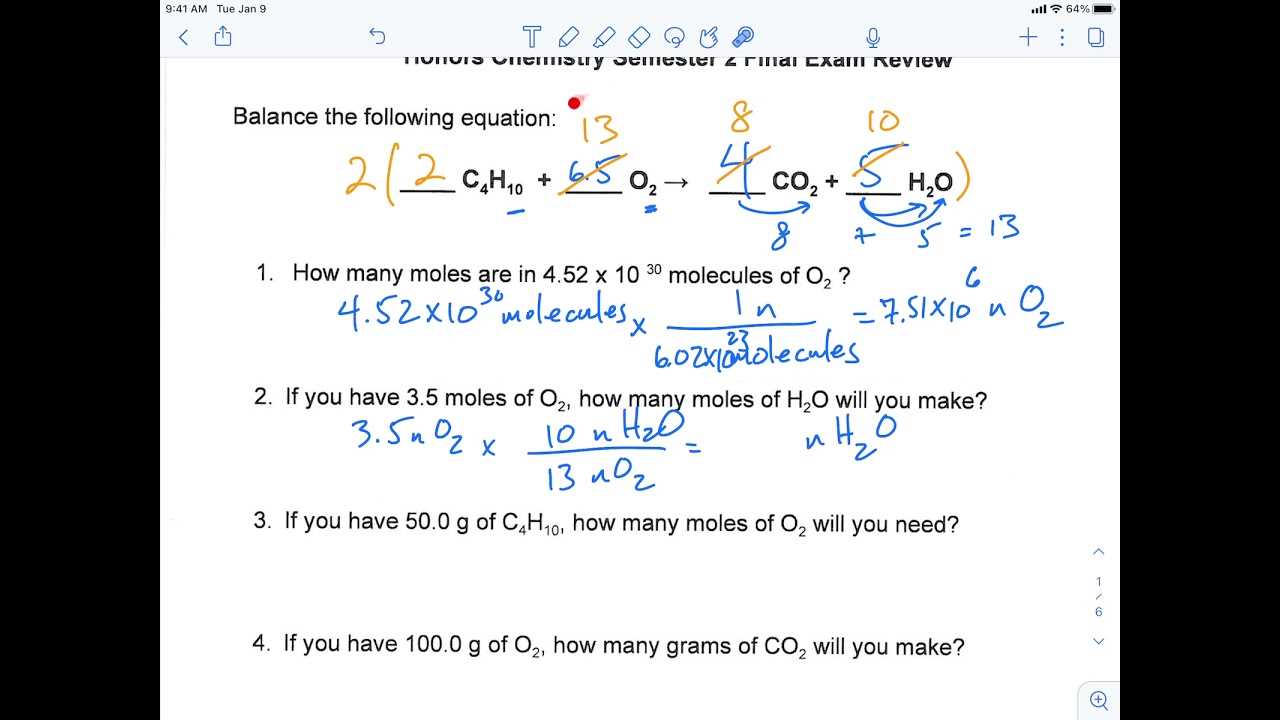
Make sure to cover the following areas to strengthen your understanding:
- Atomic Structure: Understand atomic models, subatomic particles, and how electrons are arranged in atoms.
- Chemical Bonds: Review ionic, covalent, and metallic bonding, as well as the properties of substances formed through these bonds.
- Stoichiometry: Focus on mole conversions, balancing equations, and determining the relationships between reactants and products in reactions.
- Thermodynamics: Revisit laws of thermodynamics, enthalpy, entropy, and Gibbs free energy, as well as energy changes in reactions.
- Acids and Bases: Understand the pH scale, strong and weak acids and bases, and their behavior in solutions.
Problem-Solving Strategies
When practicing problems, ensure you’re employing the following techniques:
- Balance chemical equations before attempting to solve any quantitative problem.
- Use dimensional analysis to convert between units, ensuring consistency throughout your calculations.
- Double-check your final answers by estimating the magnitude of the result to detect any major errors.
- Practice with past problems and sample questions to get familiar with the format and types of questions.
Time Management Tips
Effective time management during preparation is just as crucial as the review itself. Keep these tips in mind:
- Set specific time limits for each topic to avoid spending too much time on one area.
- Focus on understanding the underlying concepts, as this will help you approach unfamiliar questions with confidence.
- Review key formulas and concepts regularly rather than cramming the night before the assessment.
By following this checklist and ensuring comprehensive preparation, you’ll be equipped to tackle any challenges during the assessment and perform confidently.