
As the time approaches for your crucial assessment, understanding key concepts and mastering core skills becomes essential. The path to success involves a combination of theory, practice, and effective study strategies. By focusing on the right areas, you can boost your confidence and ensure that you’re fully equipped for the challenges ahead.
In this guide, we will explore the most important topics you need to review, provide useful strategies to manage your preparation, and offer tips to tackle complex problems. Whether you’re facing complex reactions or intricate calculations, this resource is designed to help you navigate through each section efficiently.
Preparation is the key, and the more focused your approach, the more successful you’ll be in understanding and applying the material. By dedicating time to practice and reviewing past questions, you can significantly increase your chances of performing well.
Preparation for the Key Assessment
When facing a challenging evaluation, mastering essential concepts and refining problem-solving techniques are the cornerstones of success. This section will guide you through the crucial topics, offering practice questions and methods to effectively tackle each section. A structured approach is vital to ensure that no aspect is overlooked.
Critical Topics to Focus On
Understanding the most frequently tested subjects is crucial for maximizing your performance. Key areas often include:
- Reaction mechanisms and the steps involved
- Thermodynamic principles and their applications
- Behavior of gases and solutions
- Acid-base equilibria and calculations
- Redox reactions and their significance
Effective Study Techniques
Proper preparation involves more than just reviewing notes. It’s about understanding how to approach problems efficiently:
- Practice regularly: Work through practice problems to strengthen your understanding.
- Review key equations: Memorize important formulas and know when to apply them.
- Simulate test conditions: Time yourself while solving problems to mimic actual test situations.
- Identify weak areas: Focus additional time on concepts that you find challenging.
Essential Concepts for Exam Success
Mastering core principles is fundamental when preparing for an important assessment. A solid grasp of key topics ensures you can approach complex problems with confidence. Focusing on foundational concepts allows you to build your knowledge progressively, giving you a strategic advantage when tackling difficult questions.
Understanding the interconnections between different areas of study can also enhance your ability to apply theoretical knowledge to practical scenarios. The ability to connect various concepts not only strengthens your understanding but also improves your problem-solving skills, making it easier to handle any challenge that arises.
Effective preparation is not just about memorizing facts, but about cultivating a deep understanding of the material, allowing you to adapt to a wide range of questions and topics. By honing your knowledge of these key areas, you are more likely to perform successfully under pressure.
How to Study Effectively for the Test

Effective preparation involves more than simply reviewing notes or memorizing facts. It requires a strategic approach that incorporates understanding key ideas, practicing problem-solving, and managing your time efficiently. A focused study routine that targets the most important concepts will provide a strong foundation for success.
Start by organizing your study sessions into manageable chunks. Prioritize areas that require the most attention, and dedicate time to mastering complex topics. Consistent practice, rather than cramming at the last minute, is essential for long-term retention and understanding.
| Study Technique | Description |
|---|---|
| Active Recall | Test yourself on key concepts to reinforce your memory and understanding. |
| Spaced Repetition | Review material at increasing intervals to improve long-term retention. |
| Practice Problems | Work through a variety of problems to gain confidence in applying concepts. |
| Concept Mapping | Create diagrams that connect related ideas to visualize relationships. |
By integrating these techniques into your study routine, you can improve both your understanding and your ability to recall information during the test. Balance your study time across different topics to ensure comprehensive preparation and avoid neglecting any crucial areas.
Key Topics Covered in General Chemistry 2
In any rigorous assessment, there are several fundamental areas that are consistently tested. Understanding these topics not only helps you grasp the material more deeply but also prepares you for the most challenging questions. A comprehensive review of these subjects is essential to ensure a strong performance when faced with complex problems.
Core areas often include concepts related to chemical behavior, molecular interactions, and the underlying principles governing reactions. Proficiency in these topics enables you to apply your knowledge to both theoretical and practical situations, providing the necessary skills to tackle a wide range of questions effectively.
Some of the key topics typically emphasized are:
- Thermodynamics and its applications
- Reaction mechanisms and kinetics
- Equilibria, including acid-base and solubility
- Electrochemistry and redox reactions
- Properties of gases, liquids, and solids
By mastering these essential areas, you’ll be prepared for any challenge that arises and able to confidently apply concepts when faced with complex scenarios. Each topic builds upon the other, making it important to develop a comprehensive understanding before moving on to more advanced material.
Mastering Chemical Reactions and Equations
Understanding how substances interact and transform is crucial for solving many complex problems. A strong foundation in reactions and their corresponding equations allows you to predict outcomes, balance formulas, and recognize patterns in various scenarios. Mastering these principles is essential for excelling in any challenging assessment.
One of the first steps to mastering reactions is learning how to balance equations correctly. This involves ensuring that the number of atoms of each element is conserved, which reflects the law of conservation of mass. Once you understand the concept, practicing different reaction types will solidify your ability to identify and predict products.
Types of reactions you should focus on:
- Synthesis and decomposition reactions
- Single and double displacement reactions
- Combustion reactions
- Redox reactions
Key techniques for mastering equations:
- Balancing coefficients: Adjust the coefficients of reactants and products to ensure atom conservation.
- Identifying reaction types: Recognize the pattern of a given reaction to apply the right method for solving it.
- Understanding stoichiometry: Use molar relationships between reactants and products to solve quantitative problems.
By continuously practicing these strategies and understanding the underlying concepts, you’ll develop the skill to solve even the most complex reactions with ease and confidence.
Understanding Stoichiometry in Depth
Stoichiometry is a fundamental concept that bridges the gap between chemical reactions and quantitative analysis. It involves understanding the relationships between the amounts of reactants and products involved in a reaction. Mastering this topic allows you to predict how much of a substance will be consumed or produced in any given process, which is crucial for solving various problems efficiently.
To fully grasp stoichiometry, you must first become familiar with the concept of molar ratios, which dictate the proportions in which substances react. Once you understand the basic principles, applying them to complex reactions becomes more straightforward, and you can calculate unknown quantities with precision.
Key techniques for mastering stoichiometry:
- Converting between units: Use conversion factors to move between moles, grams, liters, or molecules.
- Using balanced equations: A balanced reaction is essential for determining the correct molar ratios of reactants and products.
- Limiting reagents: Identifying the limiting reagent in a reaction allows you to determine the maximum amount of product that can be formed.
Practical applications of stoichiometry:
- Calculating reactant quantities needed for a desired product amount.
- Determining product yields and efficiency in industrial reactions.
- Predicting the outcomes of various reaction conditions based on initial concentrations.
By practicing stoichiometric calculations and understanding the relationships between substances, you’ll gain confidence in handling a wide variety of problem types, making it easier to solve challenges in both theoretical and practical contexts.
Tips for Balancing Chemical Equations
Balancing chemical equations is a critical skill that requires both attention to detail and an understanding of the principles behind chemical reactions. A well-balanced equation ensures that the law of conservation of mass is obeyed, meaning the number of atoms of each element remains constant before and after the reaction. Mastering this process is essential for solving a wide range of problems efficiently and accurately.
Step-by-Step Approach to Balancing
One of the most effective ways to balance equations is by following a systematic approach:
- Start with complex molecules: Begin by balancing elements that appear in fewer compounds first.
- Balance atoms one at a time: Focus on ensuring that the number of atoms of each element is the same on both sides of the equation.
- Adjust coefficients: Use whole number coefficients to balance atoms, making sure not to alter the subscripts of the compounds.
- Check the balance: After making adjustments, double-check that all elements are balanced and that the equation obeys the conservation of mass.
Common Mistakes to Avoid
- Changing subscripts: Never alter the subscripts in a compound, as it changes the identity of the substance.
- Forgetting to check oxygen and hydrogen: These elements often appear in multiple compounds, so balance them last after other elements are balanced.
- Overcomplicating the process: Don’t be afraid to simplify the equation or break it down into smaller steps to avoid confusion.
By practicing these tips and focusing on methodical steps, you’ll be able to efficiently balance equations, ensuring accuracy and improving your problem-solving skills in various contexts.
Exploring Thermodynamics in Chemistry

Thermodynamics plays a crucial role in understanding how energy flows and transforms during chemical reactions. By studying the principles of energy exchange, we can predict the feasibility and direction of reactions, as well as the conditions under which they occur. A strong grasp of thermodynamic concepts enables you to analyze both spontaneous and non-spontaneous processes, laying the groundwork for solving a wide range of scientific problems.
At its core, thermodynamics helps explain the relationship between heat, work, and internal energy within a system. Whether in the form of heat transfer or the expansion of gases, the principles of thermodynamics provide essential insight into how energy drives molecular interactions and influences reaction outcomes. Understanding these ideas also allows for the calculation of system properties, which is key to solving real-world applications, such as energy efficiency and reaction rates.
Key concepts to master:
- First Law of Thermodynamics: Energy cannot be created or destroyed, only transformed from one form to another.
- Second Law of Thermodynamics: The entropy of an isolated system always increases over time, affecting spontaneity.
- Enthalpy: The total heat content of a system, important for understanding energy changes in reactions.
- Gibbs Free Energy: A thermodynamic potential that helps predict the spontaneity of a process.
By understanding these principles and how they apply to chemical systems, you will be equipped to predict reaction behavior and energy changes accurately. This knowledge is not only vital for academic success but also for practical applications in fields like environmental science, engineering, and industrial processes.
Understanding Acid-Base Equilibria
The balance between acids and bases is essential for understanding many chemical processes, both in the lab and in nature. The dynamic equilibrium that occurs between these species plays a critical role in determining the pH of a solution and influencing the direction of various reactions. Mastering acid-base equilibria allows you to predict how a solution will behave under different conditions and understand the underlying principles governing pH changes.
Key Concepts in Acid-Base Equilibria
Acid-base equilibria are governed by several important principles that help explain how acids and bases interact in solution:
- Strong vs Weak Acids and Bases: Strong acids and bases completely dissociate in solution, while weak acids and bases only partially dissociate.
- Equilibrium Constant (Ka and Kb): The acid dissociation constant (Ka) and base dissociation constant (Kb) help quantify the strength of an acid or base in solution.
- pH and pOH: pH measures the acidity of a solution, while pOH measures the basicity. These are related by the equation pH + pOH = 14 at 25°C.
Techniques for Solving Acid-Base Problems
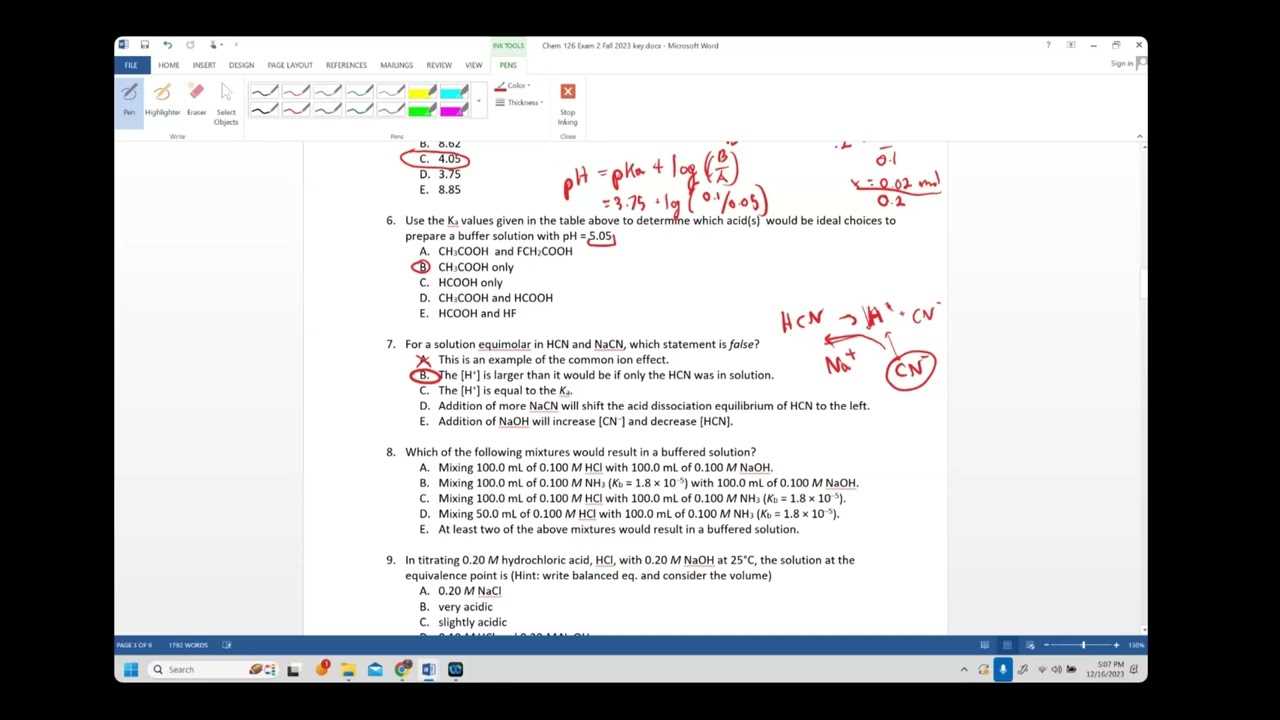
To solve acid-base equilibrium problems effectively, use the following strategies:
- Write the equilibrium expression: Set up the reaction and include the concentrations of reactants and products.
- Set up an ICE table: ICE stands for Initial, Change, and Equilibrium concentrations, which help track how concentrations change during the reaction.
- Use approximation methods: For weak acids and bases, you may approximate the change in concentration if it is small enough compared to the initial concentration.
By understanding these key concepts and applying effective problem-solving strategies, you can predict the outcome of acid-base reactions and calculate the pH and other relevant properties of solutions accurately. This knowledge is invaluable for many applications, from buffering systems in biology to industrial chemical processes.
Significance of Redox Reactions
Redox reactions are fundamental processes that involve the transfer of electrons between reactants, playing a pivotal role in a wide range of chemical, biological, and industrial applications. These reactions are responsible for energy production, corrosion, and the functioning of living organisms. Understanding redox reactions is crucial for grasping how energy is harnessed and transferred in different systems, making them essential for both scientific research and practical applications.
In redox reactions, one substance undergoes oxidation by losing electrons, while another undergoes reduction by gaining electrons. This electron exchange drives various types of processes, from the metabolism of nutrients in biological systems to the generation of electricity in batteries. Redox reactions also contribute to processes such as combustion, respiration, and even the corrosion of metals.
- Energy Production: In living organisms, redox reactions are key in processes like cellular respiration, where energy is extracted from food molecules.
- Industrial Applications: Redox reactions are central to processes such as metal extraction, electroplating, and the functioning of fuel cells.
- Environmental Impact: Redox reactions are involved in natural processes like the nitrogen cycle and are also responsible for pollution through the oxidation of pollutants.
By understanding the importance of these reactions, we can develop more efficient methods for energy conversion, improve industrial processes, and mitigate environmental damage. Mastering redox reactions provides insight into how matter interacts at the molecular level, making it a cornerstone of scientific and technological advancement.
Interpreting Reaction Kinetics and Rates
Understanding how reactions proceed over time is crucial for predicting and controlling the outcomes of chemical processes. Reaction kinetics focuses on the speed at which reactions occur and the factors that influence this rate. By studying these aspects, we can determine how changes in conditions like temperature, pressure, and concentration impact the progress of a reaction. This knowledge is vital for optimizing industrial processes, understanding biological systems, and controlling reaction pathways.
Reaction rates are influenced by multiple factors, including the nature of the reactants, the presence of catalysts, and the environmental conditions. Kinetics allows us to derive mathematical models that describe the behavior of reactions and can help predict how long a reaction will take or under what conditions it is most efficient.
Factors Affecting Reaction Rates
The rate of a chemical reaction can be influenced by several key factors:
| Factor | Effect on Reaction Rate |
|---|---|
| Concentration of Reactants | Increasing concentration typically increases reaction rate as more particles collide. |
| Temperature | Higher temperature generally increases the reaction rate by providing more energy for collisions. |
| Catalysts | Catalysts lower the activation energy, increasing the reaction rate without being consumed in the process. |
| Surface Area of Reactants | Larger surface area allows more collisions, speeding up the reaction. |
By carefully analyzing the factors that affect reaction rates, scientists can control and optimize reactions for practical applications, such as in manufacturing, drug development, and environmental processes. A solid grasp of reaction kinetics allows for precise manipulation of reaction conditions to achieve desired outcomes efficiently.
Importance of Atomic Structure Knowledge
Understanding the fundamental arrangement of matter at the atomic level is key to comprehending a wide range of chemical phenomena. The structure of atoms determines how elements interact, bond, and behave in various reactions. This knowledge provides the foundation for predicting chemical behavior, designing new materials, and understanding the properties of substances. A deep grasp of atomic structure also enhances our ability to manipulate and control reactions in diverse fields, from medicine to energy production.
At the core of atomic structure lies the nucleus, composed of protons and neutrons, surrounded by electrons in discrete energy levels. The arrangement of electrons in these energy levels governs how atoms bond and react with other atoms. This arrangement explains everything from the chemical properties of elements to their phase changes, magnetic properties, and interactions with light. Knowing how atoms bond and how their structures affect their behavior is essential in fields like nanotechnology, environmental science, and pharmaceuticals.
Key Components of Atomic Structure
| Component | Role in Atomic Behavior |
|---|---|
| Protons | Determine the element’s identity and its atomic number. |
| Neutrons | Contribute to atomic mass and influence isotopic properties. |
| Electrons | Responsible for chemical bonding and reactions through energy levels and orbitals. |
| Electron Configuration | Defines the arrangement of electrons, which dictates an atom’s reactivity and bonding behavior. |
A solid understanding of atomic structure allows for the manipulation of materials at the atomic scale, leading to advancements in numerous scientific and technological areas. Whether it’s synthesizing new compounds, designing advanced electronics, or exploring the universe at a molecular level, atomic structure knowledge serves as the cornerstone of modern science and innovation.
What You Need to Know About Solutions
Solutions are a fundamental aspect of many chemical processes and are found in a wide variety of applications, from industrial manufacturing to biological systems. At the core, a solution is a homogeneous mixture of two or more substances where one component dissolves in another. The behavior of solutions, including their formation, concentration, and properties, plays a key role in chemical reactions, medical treatments, and environmental processes.
Understanding how substances dissolve and the factors that influence solubility is essential for controlling reaction rates and designing effective processes. Whether it’s adjusting the concentration of a solution for a chemical reaction or understanding how electrolytes behave in biological systems, solutions are critical to many scientific fields.
Key Concepts of Solutions
- Solvent: The substance that dissolves the solute, typically in greater quantity.
- Solute: The substance being dissolved in the solvent.
- Concentration: A measure of how much solute is dissolved in a given amount of solvent, often expressed in molarity or molality.
- Solubility: The ability of a substance to dissolve in a solvent, which can depend on factors like temperature, pressure, and the nature of the solute and solvent.
Types of Solutions

- Solid in liquid: Common examples include saltwater and sugar dissolved in water.
- Gas in liquid: Gases like oxygen dissolved in water.
- Liquid in liquid: Alcohol dissolved in water is a typical example.
- Gas in gas: Air, which is a mixture of various gases, is an example of a solution.
By understanding the properties and behavior of solutions, you can better predict how different substances will interact, how to control their concentrations, and how to manipulate them for various practical purposes. Whether you’re studying the behavior of gases in liquids or adjusting concentrations for laboratory reactions, a solid grasp of solution dynamics is essential.
Gaining Confidence with Organic Chemistry

Mastering the complex concepts of organic compounds and their reactions can be a challenging yet rewarding experience. Understanding the structure, behavior, and transformation of organic molecules opens the door to many fascinating and practical applications in medicine, agriculture, and industry. Confidence in this area comes from consistent practice, a solid grasp of foundational principles, and the ability to connect abstract concepts to real-world scenarios.
One of the best ways to build confidence is through a methodical approach. Breaking down each topic into manageable parts allows for a deeper understanding of how individual components fit together. Focusing on core concepts such as bonding, functional groups, and reaction mechanisms is crucial for making sense of more advanced topics. Repetition is also key; regularly solving problems and working through reaction mechanisms helps reinforce learning and improve retention.
Key Strategies for Success
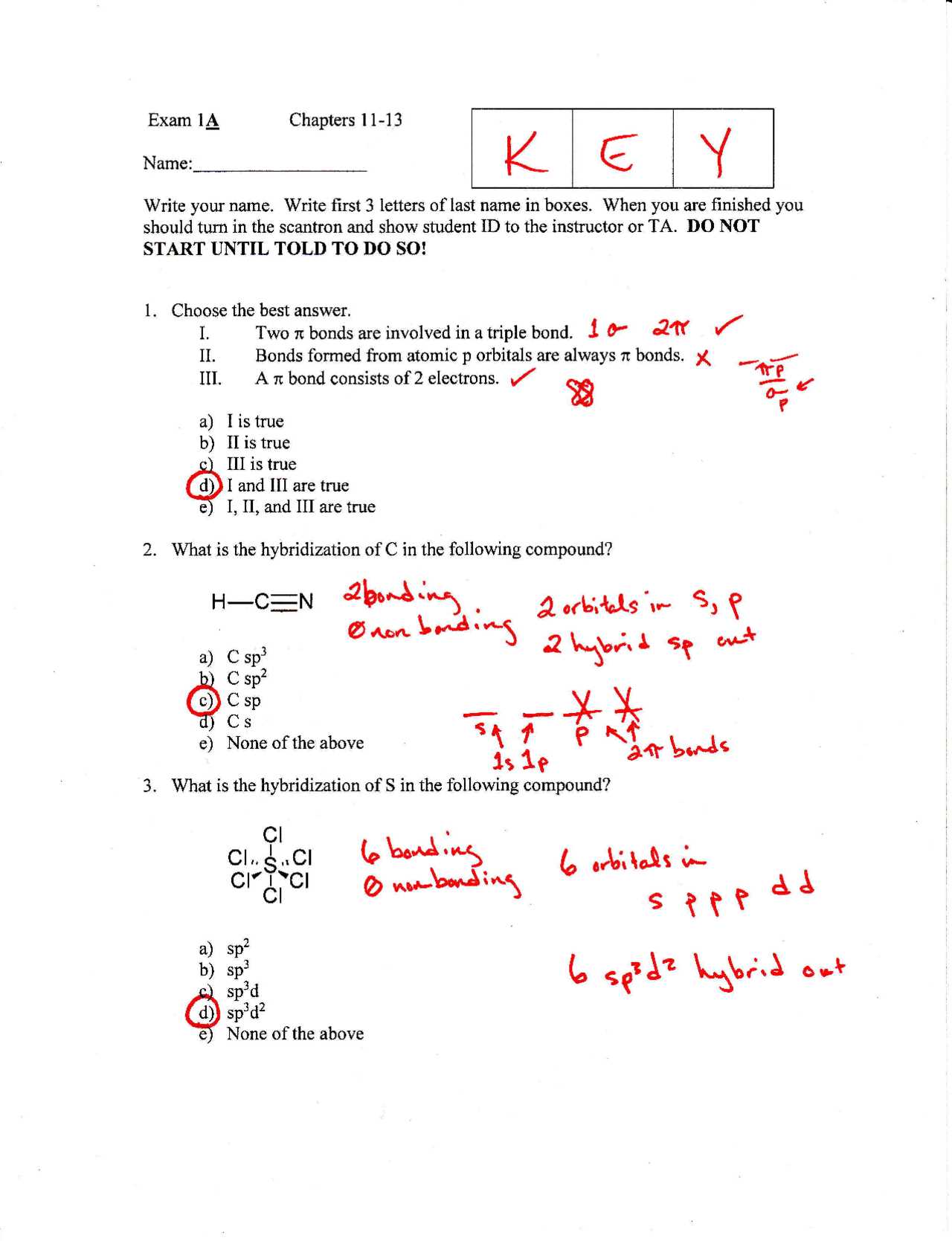
- Start with the Basics: Focus on mastering the structure and bonding of organic molecules. Understanding these basics will provide a solid foundation for more complex reactions.
- Work Through Reaction Mechanisms: Understanding the steps involved in organic reactions helps visualize how molecules change, making it easier to predict and solve problems.
- Practice Problem-Solving: Regularly practicing problems and reviewing past examples helps to solidify your understanding and boost your confidence.
- Use Visual Aids: Diagrams and molecular models can make abstract concepts more tangible and help visualize the structure of organic compounds.
By focusing on these strategies, you can gain the confidence needed to tackle even the most challenging problems in organic chemistry. Consistency, practice, and a deeper understanding of how molecules interact will help you excel and build a lasting foundation in the field.
Common Mistakes to Avoid on the Exam
Many students make similar errors during assessments, which can negatively impact their overall performance. Recognizing these frequent pitfalls and taking steps to avoid them can significantly improve your results. By understanding the most common mistakes, you can better prepare yourself and avoid unnecessary setbacks when faced with complex questions or time constraints.
One of the most prevalent mistakes is rushing through the questions without fully reading them. It’s easy to overlook important details or instructions when under pressure, leading to missed points. Another common error is neglecting to show your work, especially when solving multi-step problems. Failing to demonstrate the thought process can make it difficult to earn partial credit even when the final answer is correct.
Key Mistakes to Watch Out For
- Misreading the Question: Always read the instructions and questions carefully. Look for keywords and make sure you understand exactly what is being asked before you begin.
- Skipping Steps: Avoid jumping straight to the answer without showing your reasoning, as this may cost you points if your solution is incomplete or incorrect.
- Overlooking Units: Forgetting to include or convert units can result in incorrect calculations and deductions. Always double-check your units to ensure accuracy.
- Panic and Time Management: Losing track of time or getting anxious can lead to rushed answers or incomplete responses. Practice time management strategies during your study sessions to avoid this.
- Neglecting Review: After completing the exam, allocate time to review your answers. Many mistakes can be spotted during the final check, including careless calculation errors or skipped questions.
Avoiding these mistakes requires a combination of careful reading, methodical problem-solving, and time management. By addressing these areas, you can minimize errors and maximize your performance on the test.
Practical Strategies for Time Management
Effective time management is a key factor in achieving success, especially when working under pressure. Having a clear plan and knowing how to allocate time efficiently can help you stay focused and make the most of every moment. Whether you’re dealing with complex problems or multiple tasks, strategic planning ensures that you approach each challenge with confidence and clarity.
One of the first steps in managing time well is breaking down the task into smaller, more manageable parts. This allows you to prioritize and focus on one section at a time without feeling overwhelmed. Additionally, practicing time management during study sessions can help you gauge how much time to allocate for each type of question or topic.
Effective Time Allocation Tips
- Set Clear Priorities: Identify the most important or time-consuming sections first. Begin with the areas that are worth more points or require more attention.
- Use a Timer: Allocate a specific amount of time to each question or section. Setting a timer can prevent you from spending too long on any single task.
- Start with Easier Tasks: Quickly tackle the questions you know best to build momentum. This boosts your confidence and frees up more time for difficult problems later.
- Practice Under Timed Conditions: Simulate test conditions during your study sessions. By practicing under time constraints, you’ll get a better sense of how to pace yourself during the actual assessment.
- Review Your Progress: Periodically check the clock to ensure that you are staying on track. Adjust your pace if necessary to ensure you can complete all sections in the available time.
By applying these strategies, you can ensure that you’re using your time wisely, reducing stress, and maximizing your ability to perform well under time pressure. Effective time management is a skill that can be developed with practice, and once mastered, it will significantly improve both your productivity and your results.
Using Practice Tests to Prepare
One of the most effective ways to strengthen your understanding and boost your confidence before an assessment is through regular practice. Taking mock tests allows you to familiarize yourself with the types of questions you might encounter, refine your problem-solving skills, and improve your time management under pressure. Practice tests simulate real conditions, helping you get accustomed to the pace and format of the tasks at hand.
When utilizing practice tests, it’s important to approach them strategically. Rather than just aiming to complete them, use the opportunity to identify areas of weakness. After each session, review your answers thoroughly to understand any mistakes and correct them. This process of self-assessment helps reinforce concepts and prevent the same errors from occurring during the actual evaluation.
Maximizing the Benefits of Practice Tests
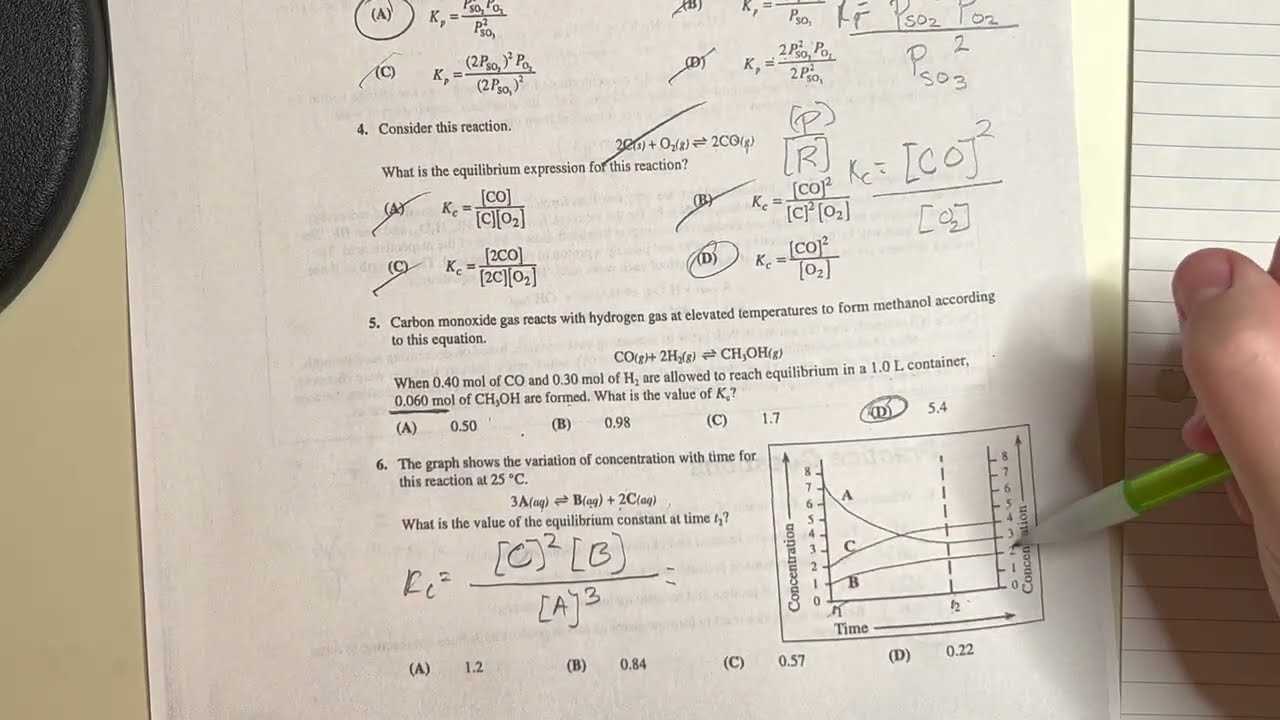
- Set a Realistic Time Limit: Simulate the time constraints you will face during the assessment. This will help you become comfortable working within a specific timeframe.
- Review Incorrect Answers: After completing each test, go through the questions you got wrong. Understanding why you made certain mistakes is crucial to improving your knowledge.
- Focus on Problem Areas: Pay extra attention to topics or types of questions where you struggled. Spend more time reviewing these sections to gain a deeper understanding.
- Take Multiple Practice Tests: The more tests you complete, the more familiar you will become with the material. Repeated exposure helps reinforce key concepts and solidifies your knowledge.
- Track Your Progress: Keep a record of your scores and note any improvements or areas that still need attention. This can provide motivation and a clear indication of where more study is required.
By incorporating practice tests into your study routine, you not only improve your familiarity with the material, but you also enhance your ability to apply concepts quickly and efficiently. The more you practice, the more prepared and confident you’ll feel when facing the actual challenges ahead.
How to Review Correct Answers Effectively
Reviewing the correct responses after completing a test or practice exercise is just as important as identifying your mistakes. This step allows you to reinforce your understanding, confirm the logic behind your choices, and solidify your knowledge. Rather than simply checking whether your answers are correct, take the time to evaluate why the solution is right and how the principles apply to other scenarios.
Effective review involves more than just marking an answer as correct. Focus on understanding the reasoning behind each correct response and how different concepts connect. This process will deepen your grasp of the material and help you retain important information for future assessments.
Steps for Effective Review
- Analyze the Reasoning: After confirming the answer is correct, examine the steps taken to reach that conclusion. Ensure you understand every aspect of the solution process.
- Apply Different Approaches: Try solving the same problem using an alternative method. This enhances your flexibility in applying knowledge and helps you understand the material from multiple angles.
- Link Concepts Together: Recognize how the correct answer relates to other topics you’ve studied. This will help you understand the broader picture and see how different concepts interact.
- Understand the Underlying Principles: Go beyond memorizing the steps. Understand the fundamental principles that support the correct answer, as this knowledge can be applied to new problems.
- Practice Similar Problems: Once you’ve reviewed the correct responses, find and work through similar questions to further reinforce the material and test your understanding.
By actively engaging with the correct answers, you will develop a deeper comprehension of the material and gain greater confidence in applying concepts. This method of review encourages long-term retention and prepares you to tackle more complex problems in the future.