
In this section, you’ll explore essential principles that govern the transformation of substances in various processes. Understanding these fundamental ideas is crucial for building a strong foundation in the subject and excelling in your studies. From the basic laws to more complex calculations, grasping the core topics will enhance your overall comprehension.
Reaction mechanisms, energy flow, and the role of different substances are some of the primary aspects that come into focus. With a deep dive into balancing equations and understanding how materials interact, you’ll gain a clearer picture of the forces at play in every reaction.
Preparation for assessments requires not only memorizing formulas and processes but also applying them to real-world scenarios. By the end of this section, you’ll be equipped with the skills to solve problems confidently and interpret results accurately.
Chemistry Matter and Change Chapter 8 Study Guide Answers
This section focuses on the key principles that govern the transformation of substances through various processes. Understanding these core concepts will help in solving problems, balancing equations, and interpreting the results of different chemical reactions. The aim is to ensure clarity on the methods used to quantify and analyze these transformations in real-world scenarios.
From calculating the amount of reactants to understanding the dynamics of energy exchange, this segment emphasizes the critical steps in approaching reaction problems. You’ll encounter techniques that are essential for grasping how substances interact and change during specific processes.
By mastering these topics, you’ll be able to confidently apply the principles to a variety of situations, enhancing both your theoretical knowledge and practical skills. This section is designed to prepare you for tackling more complex problems in the field with precision and ease.
Understanding Key Concepts in Chemistry
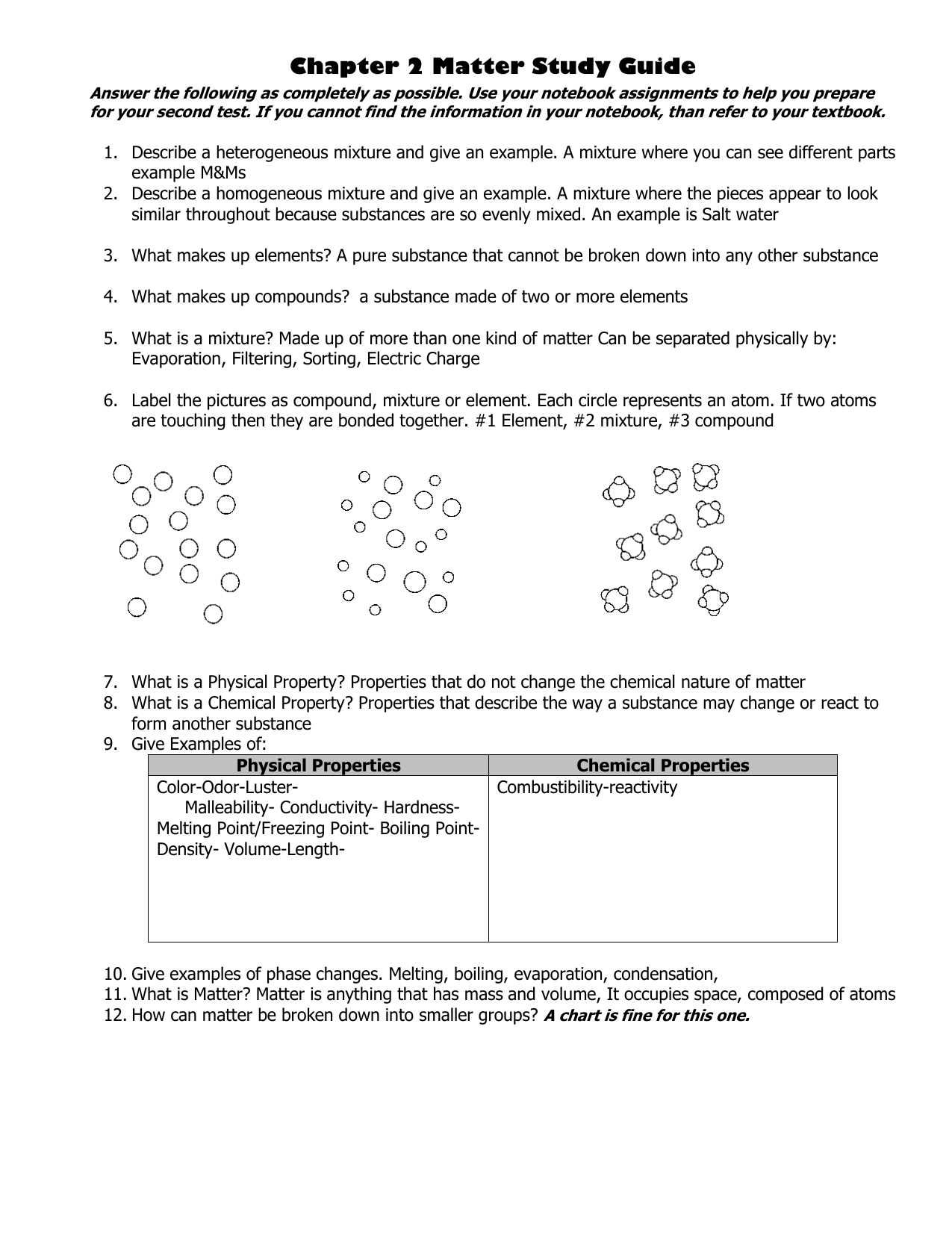
Grasping the fundamental principles that guide the transformation of substances is essential for anyone looking to understand how different materials react and interact. These core concepts form the backbone of all related topics, from basic reactions to complex phenomena. Gaining clarity on these ideas is key to solving problems and analyzing the behavior of substances under various conditions.
The Role of Reactions
Reactions involve the breaking and forming of bonds between atoms, which leads to the creation of new compounds. By recognizing the different types of processes, such as synthesis, decomposition, and displacement, you’ll be able to identify patterns and predict the outcomes of chemical interactions. Understanding how energy is absorbed or released during these events further aids in explaining their behavior.
Energy Flow and Transformation

Energy is a critical factor in many transformations, as it determines how substances change their state or undergo reactions. Whether through heat, light, or electrical energy, recognizing how energy influences these processes is vital for accurate predictions. Mastery of these concepts allows for a deeper understanding of how reactions occur and why they proceed in certain directions.
Types of Chemical Reactions Explained
Understanding the different ways in which substances interact and transform is fundamental to grasping the principles of molecular change. Various reaction types occur depending on the conditions and materials involved. Each type exhibits unique characteristics that determine the products formed and the energy changes that take place. Knowing how to classify these reactions helps predict their outcomes and understand their mechanisms.
Synthesis and Decomposition Reactions
Synthesis reactions occur when two or more reactants combine to form a single product. These reactions are essential in building complex molecules from simpler components. On the other hand, decomposition reactions involve the breakdown of a compound into simpler substances, often requiring an input of energy in the form of heat or electricity. Both types of reactions are fundamental to various chemical processes and industrial applications.
Displacement and Combustion Reactions
Displacement reactions involve one element replacing another in a compound, resulting in the formation of a new substance. This can be seen in single or double displacement reactions. Combustion reactions, on the other hand, typically involve the rapid reaction of a substance with oxygen, releasing energy in the form of heat and light. These reactions are essential in energy production and are frequently encountered in everyday life, such as in engines and fires.
Balancing Chemical Equations Simplified
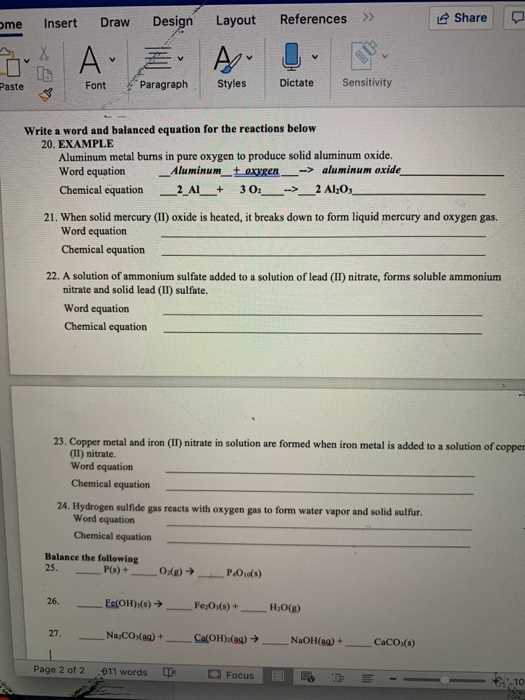
Balancing reactions is a critical skill for understanding how substances interact during transformations. The process ensures that the law of conservation of mass is upheld, meaning the amount of each element remains constant throughout the reaction. By adjusting the coefficients of the compounds involved, you can achieve a balanced equation where the number of atoms on both sides is equal. This step is vital for accurately representing the changes occurring in a chemical process.
Steps to Balance Equations
Start by writing down the unbalanced equation, ensuring all reactants and products are correctly represented. Then, examine the atoms of each element on both sides. Adjust the coefficients of the compounds to match the number of atoms, beginning with elements that appear in fewer compounds. Keep checking the balance after each adjustment to ensure all elements are accounted for. This process may require multiple iterations to achieve equilibrium.
Common Tips for Success
Start simple by balancing elements that appear in only one reactant and one product. Check your work after every step to avoid making mistakes. Often, balancing hydrogen and oxygen comes last, as they are found in multiple compounds. Patience and practice are key to mastering this skill.
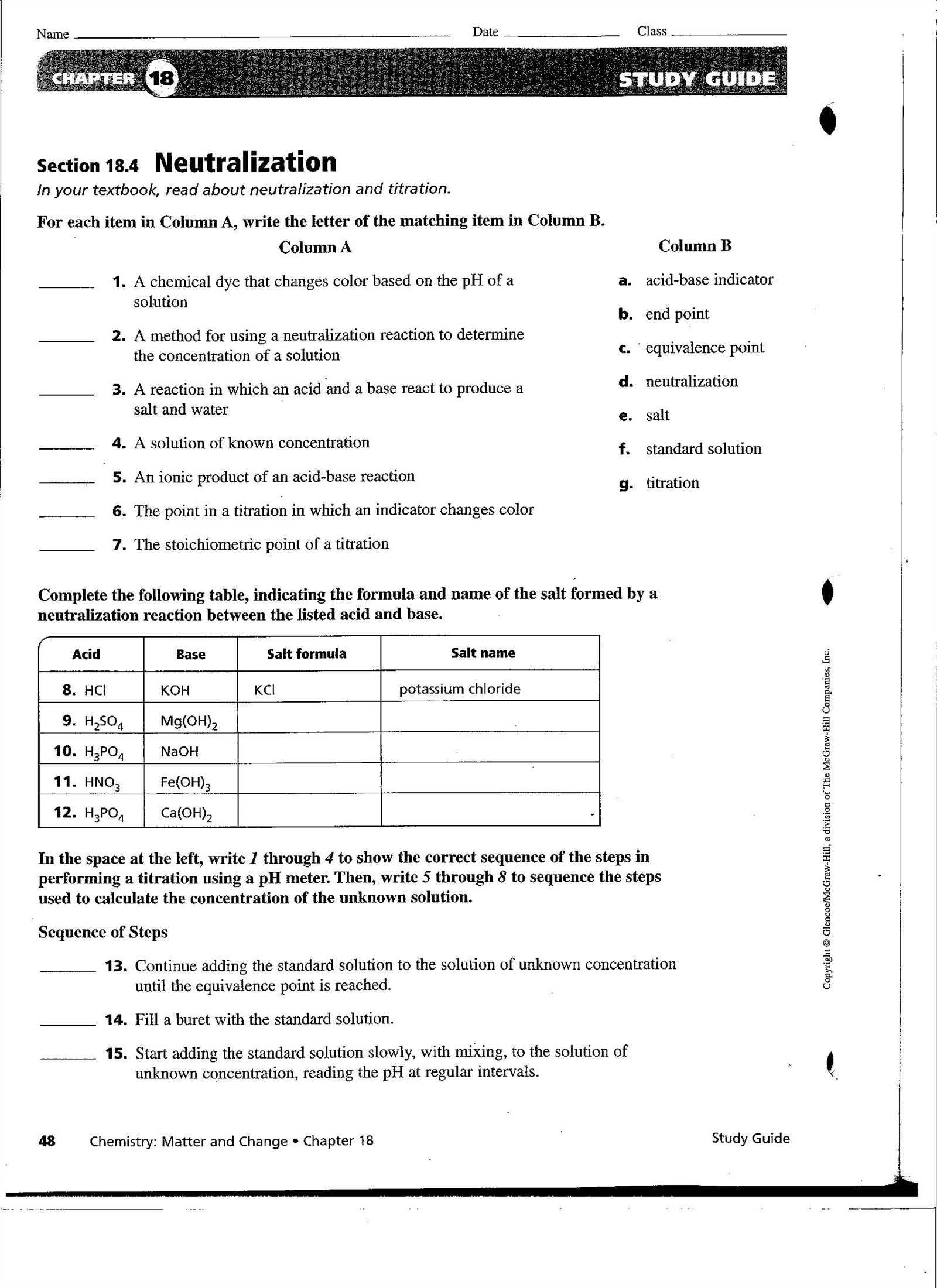
Exploring the Law of Conservation
The law governing the constancy of mass during transformations asserts that no matter how substances interact, the total amount of each element remains unchanged. This principle is fundamental in understanding why reactions occur in a particular way and how the materials involved are preserved throughout the process. Whether atoms are rearranged, bonded, or broken apart, the total number of atoms before and after the reaction is identical.
Key Points of the Law
- The total mass of the reactants is equal to the total mass of the products.
- No atoms are lost or created during a reaction, only rearranged.
- This principle is crucial for balancing chemical equations accurately.
Applications in Reactions
Understanding this principle is essential when working with various reactions. It helps in predicting the amounts of products formed and the behavior of reactants. For example, when measuring the output of a reaction in a controlled environment, you can rely on the law of conservation to ensure the results align with the expected quantities.
- In closed systems, all mass before and after a reaction should remain constant.
- Lab experiments often demonstrate how this law applies to real-world scenarios, such as combustion or synthesis reactions.
Key Terminology for Chapter 8 Mastery

To fully comprehend the processes covered in this section, it’s essential to familiarize yourself with the key terms that define the core concepts. These terms help break down complex ideas into more manageable components, making it easier to apply them in real-world scenarios. Mastery of these words will provide clarity and allow you to approach problem-solving with confidence.
Essential Terms to Know

- Reactants – The substances that undergo transformation in a chemical process.
- Products – The new substances formed as a result of a reaction.
- Stoichiometry – The calculation of reactants and products in chemical reactions based on their quantities.
- Activation Energy – The minimum energy required to start a reaction.
- Exothermic – A type of reaction that releases energy in the form of heat.
- Endothermic – A reaction that absorbs energy from its surroundings.
Important Concepts to Master
- Understanding the difference between balanced and unbalanced reactions is essential for solving problems.
- Be familiar with conservation laws, which dictate that mass and energy remain constant during reactions.
- Recognizing the role of catalysts, which speed up reactions without being consumed.
The Role of Energy in Reactions
Energy plays a crucial role in every reaction, acting as the driving force behind the transformation of substances. Whether it’s the energy needed to initiate a reaction or the energy released as bonds break and form, understanding how energy flows through a process is essential for predicting outcomes. The type and amount of energy involved determine whether a reaction will occur spontaneously or require external input.
Types of Energy Changes
There are two primary types of energy changes in reactions: exothermic and endothermic. In exothermic reactions, energy is released to the surroundings, usually in the form of heat or light. These reactions tend to occur spontaneously as they release more energy than they consume. Conversely, endothermic reactions absorb energy, requiring an external source of energy, like heat, to proceed. These reactions are common in processes like photosynthesis.
Activation Energy and Reaction Rates
Before a reaction can proceed, a certain amount of energy, known as activation energy, must be supplied to break the bonds of the reactants. This energy barrier determines the rate at which a reaction will occur. In some cases, a catalyst is used to lower the activation energy, speeding up the process without being consumed in the reaction.
Identifying Reaction Types in Chemistry

Understanding the various forms of reactions is fundamental for predicting the outcome of different processes. Each reaction type follows distinct patterns, which can help identify the specific changes occurring between reactants and products. By recognizing these patterns, one can better understand how different substances interact, and this knowledge allows for more accurate manipulation of materials in scientific and industrial settings.
There are several common reaction types, each with unique characteristics. These include reactions where substances combine, decompose, or exchange parts with one another. Additionally, energy changes during these reactions, whether energy is released or absorbed, can also provide clues to the type of reaction taking place. Recognizing the signs of each type is key to mastering this aspect of chemical processes.
Stoichiometry and Its Importance
Stoichiometry is an essential concept in understanding how different substances react and how the quantities of those substances are related. By calculating the amounts of reactants and products, this method ensures that reactions are balanced in terms of both mass and energy. Whether you’re conducting a laboratory experiment or analyzing an industrial process, stoichiometry allows for accurate predictions of the required materials and expected outputs.
Key Applications of Stoichiometry
- Determining the exact amounts of reactants needed for a reaction to proceed without excess waste.
- Calculating the expected yield of products in a reaction, which helps to optimize efficiency.
- Ensuring that reactions comply with conservation laws by maintaining a consistent balance of atoms.
Why It Matters
- Without stoichiometric calculations, it would be impossible to predict how much of each substance is involved in a reaction, leading to inefficient or incomplete processes.
- In large-scale chemical manufacturing, stoichiometry ensures that the correct proportions of materials are used, preventing costly mistakes.
Understanding Limiting Reactants Clearly

In many reactions, one substance runs out before the others, limiting how much product can be formed. The substance that gets consumed first is known as the limiting reactant. Identifying this reactant is crucial for predicting the maximum amount of product that can be produced. It helps to determine the efficiency of a reaction and ensure that no material is wasted.
How Limiting Reactants Affect Reactions
The limiting reactant controls the overall progression of a reaction. Once it is completely consumed, the reaction stops, even if other reactants are still available in excess. By calculating the amount of the limiting reactant, we can accurately predict the quantity of products produced and avoid unnecessary waste of resources.
Steps to Identify the Limiting Reactant
- Write down the balanced chemical equation for the reaction.
- Convert the amounts of each reactant into moles.
- Use stoichiometry to determine how much of each reactant is needed to react with the others.
- The reactant that is entirely consumed first is the limiting reactant.
How to Interpret Chemical Diagrams
Understanding chemical diagrams is an essential skill for visualizing how substances interact during reactions. These diagrams provide a clear representation of the components involved, helping to explain the molecular changes that occur. By interpreting these visuals correctly, one can gain insights into the behavior of different elements and compounds in a given reaction.
Chemical diagrams often use symbols, arrows, and labels to represent molecules, bonds, and reactions. Each part of the diagram conveys specific information about the process, such as the arrangement of atoms or the flow of energy. A strong grasp of these representations allows for easier prediction and analysis of reactions.
Key Elements in Chemical Diagrams
- Atoms and Molecules: Represented by symbols, these indicate the substances involved in a reaction.
- Bonds: Lines or different types of arrows show how atoms are connected or how they interact during a process.
- Reaction Pathways: Arrows often indicate the direction of transformation or energy transfer during the reaction.
Tips for Interpreting Diagrams
- Familiarize yourself with the common symbols and notation used in the diagrams.
- Pay attention to the orientation and direction of arrows to understand the flow of reactants and products.
- Look for patterns that correspond to well-known reaction types, such as combination or decomposition processes.
Moles and Their Application in Reactions
The concept of moles plays a central role in understanding how reactions occur on a molecular level. By using moles, we can quantify the number of particles involved in a reaction, whether atoms, molecules, or ions. This allows for precise measurements of reactants and products, ensuring accurate calculations and predictions in chemical processes.
Moles provide a bridge between the microscopic world of individual particles and the macroscopic quantities we handle in the laboratory. Through stoichiometric calculations, we can relate the amounts of substances involved in reactions, enabling us to predict the quantities of products formed from given amounts of reactants.
How Moles Are Used in Reactions
- Determining Reactant Quantities: Moles help calculate how much of each reactant is needed for a reaction to proceed completely.
- Predicting Product Amounts: Using the mole ratio from a balanced equation, we can predict how much product will be formed from specific quantities of reactants.
- Efficiency and Yield: Understanding the role of moles helps evaluate how efficiently a reaction occurs and if the expected yield matches the theoretical value.
Practical Applications of Moles
- In industrial processes, knowing the precise amount of a substance needed ensures cost-efficiency and minimizes waste.
- In laboratory experiments, moles are used to scale reactions appropriately, ensuring that the quantities used are safe and effective for the experiment’s goal.
Practical Applications of Reaction Rates
The speed at which reactions occur has significant implications in various fields, from industrial manufacturing to environmental processes. Understanding how quickly substances react allows for better control over processes, efficiency improvements, and safety measures. By adjusting factors like temperature, concentration, or pressure, reaction rates can be manipulated to achieve desired outcomes in a timely manner.
In practical settings, knowledge of reaction rates helps in optimizing the production of goods, controlling the efficiency of energy conversion, and minimizing harmful byproducts. Moreover, industries rely on controlling reaction rates to maintain consistency, improve product quality, and reduce costs.
Applications in Industry
- Manufacturing: By controlling reaction speeds, industries can optimize the production of chemicals, pharmaceuticals, and materials, ensuring that resources are used efficiently.
- Energy Production: In power plants and fuel cells, managing reaction rates ensures that energy is produced at optimal levels while reducing the release of waste gases.
- Food Preservation: Reaction rates play a role in processes like fermentation and spoilage, where controlling the rate of chemical changes helps extend shelf life and maintain quality.
Environmental Impact
- Pollution Control: By understanding how fast harmful chemicals break down in the environment, effective strategies can be implemented to reduce pollution.
- Biodegradation: Monitoring the rate at which organic materials decompose helps in waste management and promotes more efficient recycling processes.
The Significance of Activation Energy
The energy required to initiate a reaction plays a crucial role in determining how fast a process can occur. Activation energy represents the minimum energy needed for reactants to transform into products, affecting everything from industrial processes to biological systems. Understanding this concept helps in controlling the pace of reactions and optimizing conditions for desired outcomes.
In many cases, reactions require an energy input to overcome an initial barrier before proceeding. By altering factors such as temperature, catalysts, or pressure, activation energy can be influenced, thus controlling the reaction rate. This understanding is vital for improving efficiency, reducing energy consumption, and ensuring safety in various applications.
| Factor | Effect on Activation Energy |
|---|---|
| Temperature | Increasing temperature provides more energy to the molecules, helping them overcome the activation energy threshold faster. |
| Catalysts | Catalysts lower the activation energy, allowing reactions to proceed at a faster rate without being consumed in the process. |
| Pressure | Increasing pressure can bring reactants closer together, increasing the chances of successful collisions and reducing the activation energy required. |
Common Mistakes in Chemical Calculations
In the process of performing quantitative calculations related to reactions, it is easy to encounter errors that can affect the final result. These mistakes often stem from misunderstanding the principles, improper unit conversion, or overlooking important details during the calculation process. Identifying and correcting these common pitfalls can improve accuracy and lead to more reliable results.
Below are some frequent errors that occur during chemical computations:
- Incorrect Unit Conversion: Failing to convert between units properly can lead to significant discrepancies in calculations. Always ensure that units match and are consistent throughout the problem.
- Misbalancing Equations: An unbalanced equation leads to incorrect stoichiometric ratios, making it impossible to calculate the correct amount of reactants or products.
- Forgetting to Use Molar Mass: When calculating the number of moles from mass or vice versa, neglecting to include the molar mass of compounds is a common mistake.
- Overlooking Significant Figures: Failing to consider the appropriate number of significant figures can result in inaccurate final answers. It’s crucial to maintain the correct precision throughout all calculations.
- Improper Application of Avogadro’s Number: Confusing the relationship between moles and molecules is another source of error. Always ensure that the correct conversion factor is applied when switching between moles and molecules.
- Incorrect Use of Limiting Reactants: Ignoring the concept of the limiting reactant can lead to overestimating or underestimating the amount of product formed in a reaction.
By carefully checking each step and understanding the principles behind the calculations, these common mistakes can be avoided, leading to more accurate results in any scientific inquiry.
Tips for Preparing for Chapter 8 Exams
Preparing for exams on complex topics requires focused effort, organization, and a solid strategy. Successful preparation is not just about reviewing content, but also understanding key principles and mastering the problem-solving techniques involved. Here are some effective tips to help you prepare efficiently for your upcoming assessment.
By focusing on the following approaches, you can enhance your readiness and boost your confidence:
| Preparation Strategy | Details |
|---|---|
| Review Key Concepts | Go over important definitions, formulas, and reaction types. Familiarizing yourself with these will help you solve problems more efficiently during the exam. |
| Practice Problem-Solving | Work through as many practice problems as you can. This helps reinforce the application of concepts and enhances problem-solving skills. |
| Focus on Common Mistakes | Identify where you typically make errors and review those areas carefully. This will prevent you from repeating the same mistakes during the exam. |
| Create a Formula Sheet | Prepare a list of key formulas and conversion factors. This can help streamline your exam preparation and serve as a quick reference during the test. |
| Collaborate with Study Groups | Joining a study group can provide diverse perspectives on difficult topics and allow you to tackle problems collaboratively. |
| Time Management | Allocate specific time slots to each topic and stick to your schedule. Proper time management ensures you cover everything before the exam. |
Staying organized, practicing consistently, and reviewing your weak points will help you prepare effectively for the exam. Remember to stay calm and focused–success comes from both hard work and a clear, systematic approach.