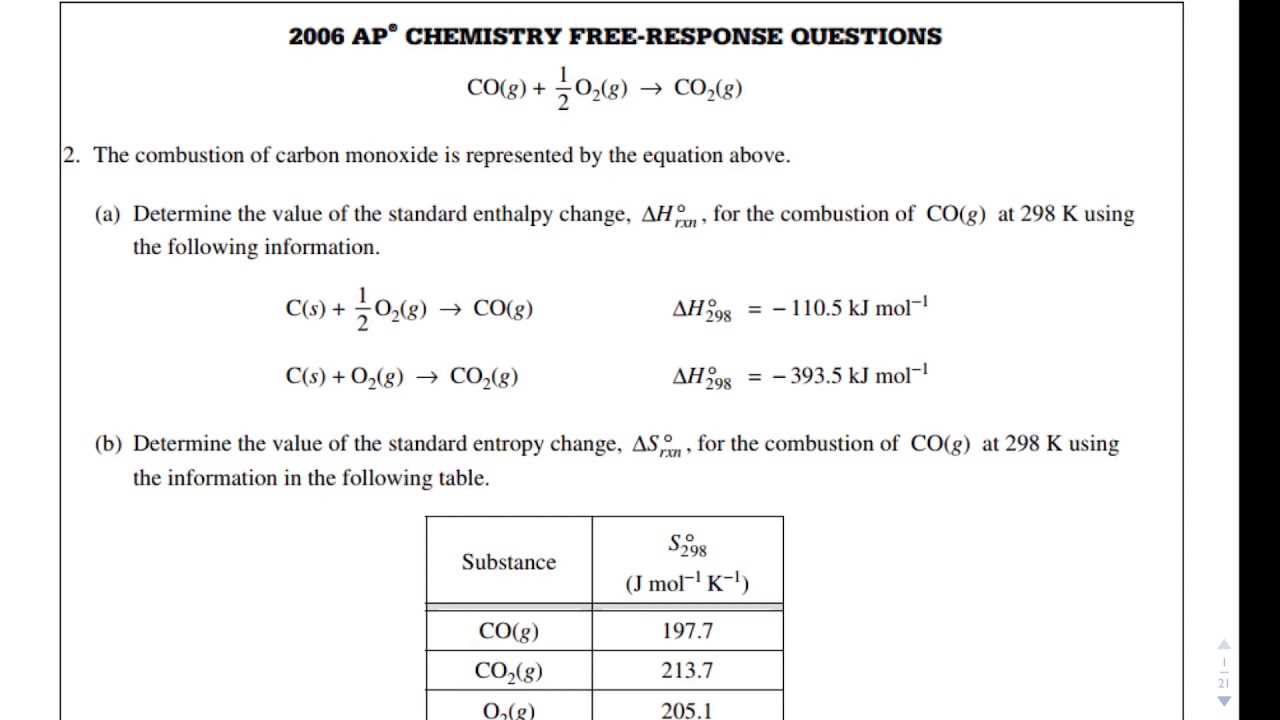
Preparing for the Advanced Placement exam in science requires focused effort and practice, especially when it comes to complex problem-solving. A key component of this preparation involves working through various types of questions that test your ability to apply knowledge in real-world scenarios. By studying past materials and exercises, students can sharpen their understanding of core concepts and improve their performance in both theory and practical aspects of the exam.
When tackling such exercises, it’s crucial to develop a strategy that not only addresses the immediate problems but also reinforces your overall understanding of the subject. Approaching each problem methodically, practicing with timed sets, and evaluating past work can significantly boost confidence. The insights gained from detailed solutions are invaluable, helping to identify recurring patterns and common pitfalls, ensuring better results on the actual test day.
Comprehensive Solutions for AP Exam Practice
In preparation for the AP science exam, it’s essential to practice with a variety of questions that require detailed explanations and in-depth application of learned material. Reviewing past problems allows students to not only test their knowledge but also refine their problem-solving techniques. Understanding the breakdown of each solution helps in mastering the skills needed to approach difficult scenarios efficiently during the actual test.
Breaking Down the Problem-Solving Process
Each problem on the exam typically involves multiple steps and requires careful thought. The key is to approach each question systematically. Here are some helpful strategies:
- Read Carefully: Take time to fully understand the question before jumping into calculations.
- Identify Key Concepts: Recognize which theories or formulas are relevant to the problem.
- Work Step-by-Step: Break down the problem into smaller, manageable parts.
- Check for Units: Ensure that all units are consistent throughout the calculation process.
- Review the Solution: Double-check for accuracy and completeness in your response.
Building Confidence Through Practice
Regular practice with previous problems not only improves accuracy but also builds confidence in tackling similar questions on test day. Familiarity with various types of problems allows students to perform more effectively under pressure. Here are some tips for practicing:
- Work in Timed Sessions: Simulate exam conditions by setting a timer for each practice session.
- Analyze Mistakes: Take the time to understand why an error was made and correct it before the next attempt.
- Use Multiple Resources: Don’t rely on a single source. Use textbooks, online resources, and past exams to gather diverse examples.
- Practice with Peers: Discuss problems with classmates to gain new perspectives and strategies.
How to Approach Free Response Questions
When tackling open-ended exam questions, it’s crucial to approach each one methodically to ensure clarity and accuracy in your response. Unlike multiple-choice questions, these require you to show your thought process, explain your reasoning, and demonstrate your understanding of key concepts. Taking a structured approach helps in organizing your thoughts and delivering well-thought-out solutions that clearly address each part of the problem.
1. Understand the Problem
Before starting your calculations or writing your explanation, take a moment to fully comprehend the question. Break it down into smaller, more manageable parts. Identify what is being asked and what information is provided. This initial step is essential to ensure you don’t overlook important details and to avoid rushing into the solution without clarity.
2. Organize Your Work
Once you have a clear understanding of the question, organize your approach. Structure your response in a way that logically flows from one step to the next. For instance, if the problem involves calculations, show all the necessary steps in sequence. If you need to explain a concept, outline your explanation before diving into details. This not only helps the grader follow your thought process but also ensures you don’t miss any key steps.
3. Be Detailed and Specific
In these types of questions, it’s important to provide detailed explanations that demonstrate your understanding. Don’t just write the final answer. Clarify how you arrived at it, and explain the reasoning behind every step. This shows the examiner that you understand the underlying principles, not just the surface-level solution.
4. Manage Your Time Effectively
Time management plays a crucial role when answering these types of questions. Allocate time to each question based on its complexity and marks. Keep track of your time, but avoid rushing through answers. Providing a well-explained response is more important than speed. If you encounter a particularly difficult question, move on and return to it later if time permits.
Overview of AP Chemistry Exam Format
The AP exam in science is designed to test a student’s understanding of fundamental principles and their ability to apply these concepts to solve complex problems. The exam consists of two main sections: multiple-choice questions and open-ended problems. Each section evaluates different aspects of knowledge, requiring both critical thinking and problem-solving skills. The overall goal is to assess a student’s depth of understanding and their ability to demonstrate this understanding under timed conditions.
1. Multiple-Choice Section
This portion of the exam includes a series of questions that test a student’s ability to recall and apply key concepts. These questions typically offer four answer choices, with one correct option. The multiple-choice section is designed to assess your knowledge of specific topics, such as atomic structure, chemical reactions, and thermodynamics. While this section requires quick thinking, it also tests the breadth of your understanding across a wide range of concepts.
2. Open-Ended Problem Section
The second section consists of several open-ended problems that require detailed, written responses. These questions are more complex and ask students to demonstrate their problem-solving abilities by showing all steps of their work and explaining their reasoning. Unlike multiple-choice questions, open-ended problems often involve calculations, explanations of chemical processes, or drawing conclusions from data. This section tests both analytical skills and the ability to articulate ideas clearly and effectively.
3. Time Management and Strategy
Given the nature of both sections, effective time management is essential for success. The multiple-choice section is designed to be completed quickly, while the open-ended problems require more time for careful thinking and detailed responses. Practicing with past exams and understanding the time constraints for each section can help students pace themselves and ensure they can address every question adequately.
Importance of Practice in Chemistry
In the realm of science exams, consistent practice plays a crucial role in building a strong foundation and improving performance. By regularly solving problems and reviewing key concepts, students can deepen their understanding and gain the confidence needed to tackle more complex tasks. This process not only strengthens retention but also sharpens problem-solving skills, enabling students to approach each question with clarity and precision.
Building Problem-Solving Skills

Solving various types of problems repeatedly helps students become familiar with different question formats and develop effective strategies for tackling them. Through practice, you learn to identify patterns, recognize important details, and apply the appropriate techniques. This hands-on experience is invaluable when faced with unfamiliar questions during the actual exam, as it enables you to think critically and solve problems more efficiently.
Enhancing Time Management
Practicing regularly also helps improve time management, a critical skill for any timed exam. By timing yourself while working through problems, you can get a sense of how long each task should take and adjust your pace accordingly. This ensures that you can allocate enough time for each question, complete the exam in full, and avoid rushing through answers, which could lead to careless mistakes.
Consistency is Key
Ultimately, consistent practice makes a significant difference in performance. Just as athletes train to perfect their skills, students should approach their studies with the same dedication. The more problems you solve and the more concepts you review, the more prepared you will be to tackle any challenges that arise during the exam.
Common Mistakes in Free Response Answers
When tackling open-ended questions, students often make a variety of mistakes that can affect their overall performance. These errors typically stem from misreading the question, neglecting to explain the thought process, or skipping critical steps in calculations. By being aware of these common pitfalls, you can avoid them and enhance the clarity and accuracy of your responses.
1. Misinterpreting the Question
One of the most frequent mistakes is misunderstanding what the question is asking. It’s essential to carefully read the entire question and identify key terms or instructions. Failing to do so can lead to providing an irrelevant answer or missing critical details.
- Not recognizing whether a calculation is required.
- Overlooking specific instructions or constraints (e.g., unit conversions).
- Failing to address all parts of the question.
2. Skipping Steps or Omiting Explanations
Another common mistake is skipping steps or neglecting to explain how you arrived at a solution. In open-ended problems, showing your work and reasoning is crucial, as it demonstrates your understanding of the underlying principles. Without a clear breakdown, you risk losing partial credit even if the final answer is correct.
- Not showing intermediate steps in calculations.
- Failing to provide reasoning behind key decisions.
- Skipping over important definitions or explanations of concepts.
3. Rushing Through the Answer
While managing time is important, rushing through the solution can lead to careless mistakes. In the hurry to complete a question, students often overlook simple errors, such as miscalculating, using incorrect units, or not checking the final answer.
- Writing incomplete or unclear solutions.
- Not double-checking the final calculations or units.
- Skipping proofreading of the response before submitting.
Breaking Down Complex Chemistry Problems
When faced with challenging scientific problems, it’s essential to break them down into smaller, more manageable parts. Tackling each component step by step not only makes the task less overwhelming but also helps ensure that no crucial details are overlooked. Whether the problem involves intricate calculations or the application of theoretical concepts, approaching it systematically can lead to clearer solutions and greater confidence in your work.
The first step in solving complex problems is to identify the key elements involved. What information is provided, and what is being asked? Once you have a clear understanding of the problem’s requirements, you can begin organizing the necessary steps and choosing the appropriate methods or formulas to apply. This structured approach reduces the chances of making mistakes and helps you stay focused on solving the problem effectively.
Another important aspect is checking for consistency throughout your solution. Always ensure that the units are correctly aligned, the steps are logically ordered, and the final solution is reasonable. Taking the time to verify your calculations and reasoning at each stage can make the difference between an accurate solution and a careless error.
Time Management Strategies for AP Exams
Effective time management is a crucial skill for performing well on any exam. With limited time to complete a variety of tasks, organizing your approach and prioritizing efficiently can make a significant difference. By managing time wisely, you can ensure that each question receives the attention it deserves, avoid rushing, and maintain clarity throughout your responses. Implementing smart strategies can help reduce stress and maximize your potential on exam day.
1. Allocate Time Based on Question Difficulty
Not all questions are created equal, so it’s important to allocate time according to their complexity. Start by quickly scanning the entire exam to assess the level of difficulty of each question. For the multiple-choice section, aim to move through the questions quickly, saving more time for open-ended problems that require detailed explanations or calculations. For longer, more complex questions, budget additional time to carefully work through each step.
- Set a timer for each section to keep track of time.
- Prioritize easier questions to gain momentum and build confidence.
- Ensure that more time-consuming questions are addressed after quicker ones.
2. Practice with Timed Mock Exams
One of the most effective ways to improve time management is through practice. Simulate real exam conditions by taking mock exams under timed conditions. This helps you get a sense of how long you can afford to spend on each type of question. It also helps you identify any time-draining habits, allowing you to make adjustments in your strategy before the actual exam.
- Use past exams to familiarize yourself with the format and question types.
- Practice under timed conditions to develop your pacing skills.
- Review your responses after practice exams to pinpoint areas where you spent too much or too little time.
Effective time management is about balancing speed with accuracy. By practicing good time allocation and refining your pacing, you’ll be better prepared to approach each section with confidence and perform at your best.
Tips for Writing Clear Chemistry Responses
Clarity is essential when responding to open-ended questions in any scientific exam. A well-written response not only demonstrates your understanding of key concepts but also helps the examiner follow your thought process easily. By presenting your ideas in a logical, step-by-step manner, you increase the chances of earning full credit for your work. Below are some effective strategies for writing clear and concise explanations in scientific exams.
1. Structure Your Answer Clearly
Organize your response into logical sections that reflect the problem-solving steps. Start by identifying the key components of the question, then proceed systematically. Whether you are performing calculations or explaining a process, clearly label each step. If needed, break down complex ideas into simpler parts and make sure to address each aspect of the question without skipping over important details.
- Use bullet points or numbered lists when appropriate to show each step clearly.
- Highlight important variables or values to make them stand out.
- Ensure your writing flows logically from one idea to the next.
2. Be Concise and to the Point
While it’s important to be thorough, avoid unnecessary details that could confuse or distract from your main points. Use precise language to express your thoughts, and refrain from adding irrelevant information. Focus on what’s directly asked and ensure that each sentence contributes meaningfully to your explanation.
- Avoid over-explaining or repeating points unless necessary.
- Be mindful of the word count and avoid lengthy, off-topic descriptions.
- Stick to the key concepts required by the question, and don’t deviate from the topic.
3. Show All Your Work
For any calculations or detailed problem-solving, it’s crucial to show all the steps involved. Even if you arrive at the correct final answer, missing intermediate steps can result in lost points. Demonstrating your method gives the examiner insight into your thought process and can earn you partial credit for the work you did.
- Write out each formula used and show how you derived the results.
- Include units where applicable to show that you’re keeping track of measurements properly.
- Check your final answer for consistency and correctness before submitting.
By following these guidelines, you can craft responses that not only display your knowledge but also make it easier for the examiner to assess your understanding. Clear, concise, and well-structured responses will always stand out in any scientific examination.
Understanding the Grading Rubric for Free Responses
In any examination, understanding the grading criteria is essential to crafting responses that align with the expectations of the evaluators. By familiarizing yourself with the rubric, you can better target the aspects of your answer that will be rewarded, ensuring that your solution is complete and accurate. The rubric provides a clear structure, highlighting the key components that will earn you points and helping you focus on the most critical aspects of the question.
1. Identifying Key Evaluation Criteria
The grading rubric typically breaks down a response into several categories, each with specific elements that are evaluated. These categories may include clarity of explanation, correct use of scientific concepts, accuracy of calculations, and the logical flow of your reasoning. By understanding how each of these factors is weighted, you can tailor your answer to meet the requirements of the rubric.
- Correctness of calculations and formulas used.
- Clarity in explaining concepts or steps.
- Proper organization and logical progression of your answer.
2. Maximizing Points with Detailed Explanations
Rubrics often emphasize the importance of showing detailed reasoning for your answers. Even if your final answer is correct, failing to demonstrate how you reached that conclusion can result in partial credit. Be sure to break down each part of the problem and provide explanations for your decisions, calculations, or reasoning. This not only ensures that you earn points for each step but also makes it easier for the grader to follow your thought process.
- Always show intermediate steps in complex calculations.
- Explain the rationale behind each decision you make in the solution.
- Provide clear definitions or concepts when needed to reinforce your answer.
3. Addressing All Parts of the Question
A common reason for losing points is not addressing every aspect of the question. Grading rubrics often specify that all parts of a question must be answered to receive full credit. Be sure to carefully read the question and ensure that you have answered every part, no matter how small. Even minor details, such as units or assumptions, can be important.
- Read each part of the question carefully and respond to each section individually.
- Ensure all units and values are consistent throughout your answer.
- Double-check that you have covered both theoretical explanations and practical calculations, if applicable.
By understanding and aligning with the grading rubric, you can craft well-structured and thorough responses that maximize your score. Focusing on accuracy, clarity, and completeness ensures that your work meets the standards expected by the evaluators, improving your performance on the exam.
Key Topics Covered in AP Chemistry 2000
When preparing for advanced scientific examinations, it’s essential to focus on the key topics that will be tested. Understanding the core concepts allows you to build a strong foundation for problem-solving and apply your knowledge to a variety of scenarios. The syllabus for this specific exam includes a wide range of fundamental principles that cover both theoretical and practical aspects of the subject. Mastering these topics ensures that you are well-equipped to handle the different types of questions that may arise during the test.
1. Atomic Structure and Properties
One of the foundational topics in this field is understanding the structure of atoms and how their properties influence chemical reactions. This includes studying atomic theory, electron configurations, periodic trends, and how these concepts can be applied to predict the behavior of substances in different conditions.
- Atomic models and theories
- Electron configuration and periodicity
- Trends in atomic size, ionization energy, and electronegativity
2. Chemical Bonding and Molecular Structure
Another critical area focuses on how atoms bond to form molecules and compounds. This involves exploring ionic and covalent bonding, molecular geometry, and the forces that influence molecular interactions. Understanding these concepts is crucial for predicting the properties of materials and their reactivity.
- Ionic, covalent, and metallic bonding
- Lewis structures and VSEPR theory
- Intermolecular forces and their effects on physical properties
3. Thermodynamics and Kinetics
Thermodynamics and kinetics are vital for understanding how chemical reactions occur and how energy changes during these processes. This includes studying enthalpy, entropy, Gibbs free energy, and the factors that affect reaction rates, such as temperature, concentration, and catalysts.
- Laws of thermodynamics
- Enthalpy, entropy, and Gibbs free energy
- Reaction rates and mechanisms
4. Equilibrium and Le Chatelier’s Principle
Equilibrium is a crucial concept in understanding reversible reactions and how systems respond to changes in concentration, temperature, and pressure. Le Chatelier’s principle plays a key role in predicting the direction of equilibrium shifts and how they affect the concentrations of reactants and products.
- Dynamic equilibrium and equilibrium constants
- Le Chatelier’s principle
- Calculations involving equilibrium concentrations
5. Acids, Bases, and Buffers
Understanding acid-base chemistry is essential for many aspects of science, particularly in fields such as biology and environmental science. This section covers the properties of acids and bases, pH calculations, titrations, and the role of buffers in maintaining pH stability.
- Strong and weak acids and bases
- pH, pOH, and pKa calculations
- Buffer solutions and their applications
These topics form the foundation of the curriculum, and mastering them is crucial for success in the exam. They encompass a broad range of concepts that can be applied to solve real-world problems, making them an integral part of your preparation strategy.
How to Use Past Answers for Study
Using previous exam responses as a study tool can be one of the most effective ways to prepare for upcoming assessments. These materials provide a wealth of examples that demonstrate how to structure your responses, what to focus on in each question, and how to approach complex problems. By reviewing past solutions, you can identify common themes, refine your problem-solving strategies, and improve your overall understanding of the subject.
1. Review the Structure and Format
One of the first things you should do when using past materials for studying is to focus on the format and structure of the solutions. Many questions follow a similar pattern, with specific sections that require detailed explanations, calculations, or logical reasoning. Understanding the expected format helps you plan your own responses effectively and ensures you meet the necessary criteria to maximize your score.
- Identify common question types and their structure.
- Learn how to break down answers into clear, concise sections.
- Note how explanations are organized and supported with examples or calculations.
2. Analyze Mistakes and Correct Them
Past solutions often provide insight into common mistakes or pitfalls that students fall into. By analyzing these errors, you can avoid making the same mistakes in your own work. Pay close attention to areas where the previous answers may have been incomplete, incorrect, or lacking in detail. This will give you a clearer idea of where to focus your studies and how to strengthen your understanding of key concepts.
- Look for incorrect calculations or missing steps.
- Identify vague or insufficient explanations and refine them.
- Practice writing clear, complete responses by correcting these errors in your own solutions.
Using past responses as a study tool not only helps reinforce your knowledge but also builds your confidence in answering complex questions under exam conditions. By mastering the structure, avoiding common mistakes, and understanding the reasoning behind each solution, you can improve both the quality and efficiency of your preparation.
Reviewing Chemical Equations in Free Response
When preparing for assessments that require in-depth written explanations, reviewing the structure and application of chemical reactions is crucial. Properly balancing and interpreting these equations not only helps in solving problems but also demonstrates your understanding of the principles governing chemical processes. A clear approach to formulating and analyzing reactions can significantly improve the accuracy of your responses.
1. Understanding the Basics of Chemical Equations
Before diving into complex problems, it’s essential to have a solid understanding of how to write and balance chemical equations. This includes knowing the symbols for elements, common compounds, and understanding how atoms are conserved during reactions. Recalling reaction types (synthesis, decomposition, combustion, etc.) and identifying the correct stoichiometric coefficients are foundational skills that are often tested in assessments.
| Type of Reaction | Example | Balanced Equation |
|---|---|---|
| Synthesis | 2H₂ + O₂ → 2H₂O | 2H₂ + O₂ → 2H₂O |
| Decomposition | 2H₂O₂ → 2H₂O + O₂ | 2H₂O₂ → 2H₂O + O₂ |
| Combustion | CH₄ + 2O₂ → CO₂ + 2H₂O | CH₄ + 2O₂ → CO₂ + 2H₂O |
2. Common Pitfalls in Writing Equations
One common challenge when dealing with chemical equations is ensuring that all elements are correctly represented on both sides of the equation. This requires not only balancing the atoms but also accounting for charge balance in ionic reactions. Many students forget to include phase indicators (solid, liquid, gas, aqueous) or overlook the requirement for stoichiometric coefficients. These small oversights can lead to incorrect solutions and lower scores.
- Double-check the phase symbols for each substance involved.
- Ensure that all coefficients are the smallest whole numbers.
- Review the law of conservation of mass to avoid mistakes in atom balancing.
By refining your approach to chemical equations, you can confidently address complex problems and demonstrate your proficiency in fundamental concepts. Regular practice with equation-based questions will further solidify your ability to apply these principles under exam conditions.
Memorization vs Understanding in AP Chemistry
In preparation for challenging assessments, it’s important to strike a balance between memorizing key concepts and deeply understanding their applications. Rote memorization may help in recalling facts quickly, but true mastery comes from grasping the underlying principles that govern reactions and processes. Understanding allows for the application of knowledge to unfamiliar scenarios, while memorization can limit the ability to think critically when faced with complex problems.
1. The Role of Memorization
Memorizing certain fundamental concepts is essential, especially when dealing with vast amounts of information. Key formulas, the periodic table, and common reaction mechanisms are examples of elements that benefit from being committed to memory. However, memorization alone does not guarantee success if you cannot apply this knowledge effectively in various contexts.
- Periodic table trends (atomic radius, ionization energy, etc.)
- Common reaction types (acid-base, redox, etc.)
- Empirical formulas and molecular weights
2. The Power of Understanding

True understanding, on the other hand, enables you to connect disparate pieces of information, recognize patterns, and apply concepts to new situations. By understanding the “why” behind reactions and processes, you can solve unfamiliar problems with greater confidence. This deeper level of learning not only helps during exams but also reinforces the ability to think critically in real-world scenarios.
- Knowing why certain reactions occur under specific conditions.
- Understanding the impact of molecular structure on reaction rates and equilibrium.
- Applying principles of thermodynamics and kinetics to predict reaction outcomes.
While memorization is useful for quick recall, understanding provides the foundation for critical thinking and problem-solving. A balanced approach, where both memorization and understanding complement each other, is key to succeeding in rigorous assessments.
How to Improve Your Answer Accuracy
Achieving precision in written assessments requires more than just knowledge; it demands a strategic approach to problem-solving and communication. Accuracy in your responses can be greatly enhanced by practicing clear reasoning, organizing your thoughts, and focusing on detail. By developing a methodical approach and avoiding common pitfalls, you can significantly improve the accuracy of your solutions.
1. Understand the Question Thoroughly
Before attempting to write any solution, ensure that you fully understand what the question is asking. Break the problem down into its core components and identify the key information. This helps prevent errors that come from misinterpreting the problem or overlooking essential details.
- Read the question multiple times to ensure you don’t miss any key details.
- Highlight important information or numbers that you will need to reference in your solution.
- Clarify the units of measurement or quantities involved to avoid confusion later.
2. Work Through the Problem Step-by-Step
Rushing through a problem can lead to mistakes, especially when complex calculations are involved. Take your time to approach the problem methodically, breaking it down into smaller, manageable steps. This will help ensure that no crucial details are skipped and that your solution follows a logical progression.
- Start with the simplest steps and build up to more complex calculations.
- Use the appropriate formulas and concepts at each stage.
- Double-check each calculation as you progress to catch any potential errors early.
3. Double-Check Your Work
Once you have finished your solution, it’s essential to review your work. Look over your calculations, check for mathematical errors, and verify that your solution makes sense. Often, errors are simple, such as misplaced decimal points or incorrect unit conversions, but they can significantly impact the accuracy of your response.
- Revisit your final answer and ask yourself if it is reasonable based on the context of the problem.
- Verify your units to ensure consistency and correctness throughout your solution.
- Consider alternative methods of solving the problem to see if you arrive at the same result.
Improving accuracy is a gradual process, built on clear understanding, careful problem-solving, and diligent checking. By adopting these strategies, you can increase the precision of your solutions and perform better in assessments.
Commonly Asked Questions on Free Response
When preparing for assessments that involve writing detailed solutions, students often have several questions regarding the best approach and strategies. These questions typically revolve around how to structure responses, what information is essential to include, and how to ensure clarity and precision in each answer. Addressing these common queries can provide students with the tools to tackle complex problems effectively and confidently.
How Should I Organize My Answer?
One of the most frequently asked questions is how to best structure a response to ensure clarity and completeness. A well-organized answer not only helps the reader understand the solution, but it also ensures that no crucial steps are missed. Here are some tips for organizing your work:
- Start by clearly identifying the given information and what needs to be determined.
- Use bullet points or numbered steps to outline your process and the formulas used.
- End with a clear, concise final answer, making sure to include proper units and significant figures.
What If I Don’t Know How to Begin?
It’s common to feel unsure about how to start when faced with a challenging problem. In these situations, it’s helpful to break down the problem into smaller, more manageable parts. Here’s how you can approach the question:
- Look for key information that can guide your first step, such as formulas, constants, or relationships between variables.
- Consider drawing diagrams or writing out known equations to help visualize the problem.
- Think about what the problem is asking for and how you might solve it step by step, even if you aren’t sure of every detail at first.
By answering these frequently asked questions and adopting a methodical approach to problem-solving, students can improve their ability to address complex problems confidently and effectively.
Boosting Confidence with Practice Tests
Preparing for any challenging exam requires consistent effort and the right strategies. One of the most effective ways to build confidence and test readiness is through practice exams. These mock tests simulate real exam conditions, helping you familiarize yourself with the format, time constraints, and types of problems that may appear. By actively engaging with practice materials, you can not only reinforce your understanding of key concepts but also identify areas that require more attention.
Practice tests serve as an essential tool for reducing exam-related anxiety. When you take mock exams under timed conditions, you can improve your time management skills, enabling you to complete the actual test more efficiently. In addition, repeated exposure to practice questions allows you to get comfortable with the testing environment, ensuring that you approach the exam with confidence.
Key Benefits of Practice Exams
Here are some important reasons why practice tests should be an integral part of your study routine:
- Familiarity with the Exam Format: Practice exams mirror the structure of the actual test, allowing you to get used to the types of questions you’ll face.
- Identifying Weak Areas: Practice tests help you pinpoint areas of weakness, so you can focus your efforts on improving your understanding of those topics.
- Time Management: Taking practice tests under timed conditions helps you develop strategies for managing your time effectively during the real exam.
- Reducing Anxiety: The more you practice, the more comfortable and confident you become, ultimately reducing exam stress.
How to Maximize the Benefits of Practice Tests
To make the most of your practice exams, follow these tips:
| Tip | Description |
|---|---|
| Simulate Real Exam Conditions | Take practice tests in a quiet environment, with a timer, and without any external help, just like the actual exam. |
| Review Your Mistakes | After completing a practice test, thoroughly review any mistakes to understand why they occurred and how to avoid them in the future. |
| Focus on Weak Areas | Use the results from your practice exams to identify topics you need to review more thoroughly. |
Incorporating regular practice exams into your study routine will not only boost your confidence but also enhance your ability to perform well on the actual test. By becoming more familiar with the exam format and practicing consistently, you will be better prepared to tackle the real challenges when the time comes.