
Preparing for advanced science exams requires a deep understanding of key concepts and the ability to apply them in complex situations. In this guide, we will explore how to effectively tackle the challenging questions presented in a well-known assessment. The exam tests your ability to think critically and solve problems, so mastering these skills is essential for success.
By examining past exam problems, students can identify recurring themes and refine their approach. This includes focusing on the core areas tested and learning the most effective strategies for organizing and articulating solutions. Focusing on clear reasoning and concise explanations will help you answer even the most intricate questions confidently.
In the following sections, we will break down various problem types, providing detailed explanations to ensure a thorough understanding of each topic. With practice and the right preparation, you can significantly improve your performance and approach the exam with greater assurance.
AP Exam Problem-Solving Strategies
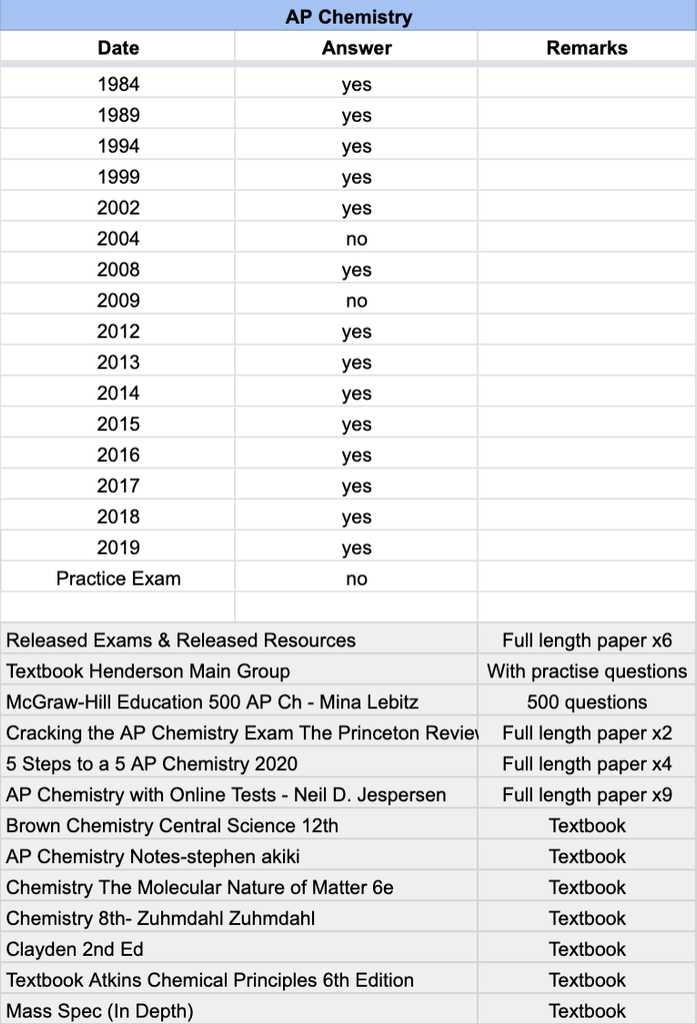
In the advanced science assessment, students are challenged with a series of complex problems that require both critical thinking and practical knowledge. Success in this part of the test depends on your ability to analyze given situations, apply theoretical principles, and explain your solutions clearly and logically. This section focuses on breaking down some of the most intricate problems from past exams to help you develop a strategy for approaching similar questions.
Breaking Down Complex Problems
Each problem presents its own set of challenges, but many can be tackled using similar methods. It’s important to identify the key concepts involved and how they relate to one another. By isolating the most relevant information and systematically working through the problem, you can reduce the complexity and find the most efficient path to the solution. Approach each problem methodically to ensure that no important detail is overlooked.
Effective Solution Techniques
Once the problem is understood, focus on presenting your reasoning in a clear, structured manner. Being concise yet comprehensive in your explanations will demonstrate your understanding and help you earn full marks. Pay attention to unit conversions, balancing equations, and logical flow to strengthen your responses. In addition, practicing under timed conditions can help improve your speed and accuracy when answering similar questions on test day.
Overview of AP Exam Format
The advanced science test is designed to assess both theoretical knowledge and practical problem-solving skills. It is divided into two main sections: multiple-choice questions and a series of open-ended tasks. The format requires students to demonstrate their ability to apply key concepts in real-world scenarios, often under timed conditions. Understanding the structure of the exam and its components is crucial for developing an effective study plan and improving performance.
Multiple-Choice Section
The first part of the exam consists of multiple-choice questions, which test a wide range of topics. These questions are designed to evaluate a student’s recall of fundamental concepts as well as their ability to apply those concepts to various situations. The multiple-choice section is typically scored automatically, making it a more straightforward part of the test.
Open-Ended Tasks
The second section focuses on more detailed, open-ended problems. These tasks require students to show a deeper understanding of the material by providing well-reasoned explanations, calculations, and logical steps. These types of questions often involve analyzing data, solving complex equations, or explaining scientific phenomena in detail. Time management and clear communication are key to performing well in this section.
How Open-Ended Questions Are Scored
The open-ended section of the exam is scored based on a detailed rubric that evaluates both the accuracy and clarity of your responses. Scorers look for well-organized answers that demonstrate a clear understanding of the concepts being tested. Each problem is broken down into several components, with partial credit awarded for correct steps even if the final solution is incorrect. A thorough and logical explanation is as important as getting the correct answer.
Scoring Criteria
Each open-ended problem is scored by looking at key elements such as the application of relevant principles, mathematical accuracy, and the logical progression of thought. The scoring guide also considers how well you justify your steps and whether you present your work clearly and coherently. Correctly identifying key variables, setting up equations, and explaining your reasoning are all crucial to earning full marks.
Partial Credit Opportunities
Even if you don’t reach the final correct solution, it’s still possible to earn partial credit for the work you do. Scorers give credit for properly executed steps that lead towards solving the problem, as long as they show correct application of underlying principles. This system rewards effort and understanding, making it essential to demonstrate your thought process throughout the task.
Key Topics in AP Exam
The advanced test covers a broad range of subjects, with certain areas consistently appearing as focal points in past assessments. These core topics are critical for building a strong foundation and tackling the more complex questions that appear on the exam. Understanding these concepts in depth will help students excel and feel more confident in their problem-solving abilities.
Thermodynamics and Energy
One of the most important areas to focus on is thermodynamics, which examines energy transformations, heat flow, and the principles governing reactions. Students are expected to understand concepts such as enthalpy, entropy, and Gibbs free energy, and be able to apply them in various contexts. Mastering these principles is essential for solving both conceptual and calculation-based problems.
Equilibria and Reactions
Another key focus is the study of chemical equilibria, where learners must demonstrate their ability to apply Le Chatelier’s principle, calculate equilibrium constants, and analyze reactions in different conditions. This topic is closely related to reaction kinetics and the mechanisms that govern the rates at which reactions occur. Being able to predict the behavior of systems at equilibrium is a skill that will be tested frequently in the exam.
Strategies for Tackling Open-Ended Questions
Approaching complex problems in an organized and systematic way is key to succeeding in the open-ended section of the exam. These types of questions often require a step-by-step approach that demonstrates both understanding and logical reasoning. By following a few simple strategies, students can increase their chances of earning full credit, even for the most challenging tasks.
Step-by-Step Approach
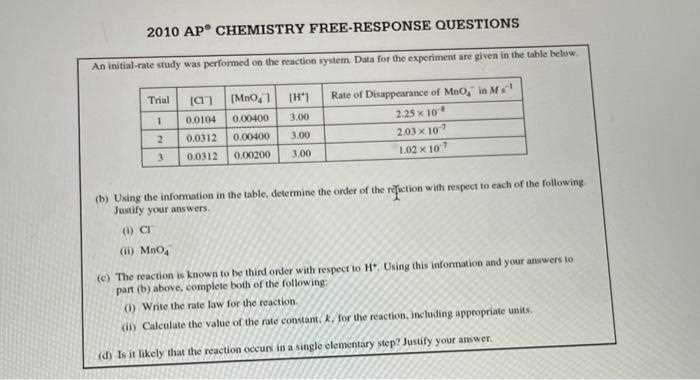
One of the most effective strategies is to break down each question into smaller, manageable parts. This allows you to tackle the problem methodically and ensures that you don’t miss any critical details. Below is a simple table outlining an effective approach to these questions:
| Step | Action | Purpose |
|---|---|---|
| 1 | Read the question carefully | Understand what is being asked and identify key concepts |
| 2 | Identify known and unknown variables | Organize your information and focus on what needs to be solved |
| 3 | Write down relevant equations | Provide a foundation for your calculations and reasoning |
| 4 | Perform calculations and explain your steps | Demonstrate both accuracy and clarity in your work |
| 5 | Double-check your solution | Ensure that no details were overlooked and that the answer makes sense |
By following this method, you ensure that each part of the question is addressed thoroughly. Clear organization is vital for conveying your thought process and for maximizing the points awarded for each task.
Common Mistakes in AP Exam Responses
While tackling complex problems, students often make mistakes that can cost them valuable points. Recognizing and avoiding these errors is crucial for improving your performance in the exam. Many of these common mistakes stem from misunderstandings of concepts, poor time management, or failure to follow logical steps in the solution process. Being aware of these pitfalls can help you approach the exam with more confidence and accuracy.
Conceptual Errors
Many mistakes arise from a lack of understanding of key principles or misapplication of formulas. These types of errors can lead to incorrect answers, even if the steps leading up to the solution are executed properly. Here are some common conceptual mistakes:
- Confusing different types of reactions (e.g., redox vs. acid-base)
- Incorrectly applying stoichiometric ratios in calculations
- Misunderstanding equilibrium and how it shifts in response to changes
- Overlooking unit conversions during problem solving
Procedure and Calculation Mistakes
Even when students understand the theory behind a question, they often make errors in the way they perform calculations or structure their responses. Here are a few common procedural mistakes:
- Skipping intermediate steps when solving equations, making it harder for graders to follow the reasoning
- Failing to balance chemical equations properly
- Not showing all work or clearly indicating which steps led to the final answer
- Omitting significant figures or using incorrect precision in final answers
By staying mindful of these common mistakes, students can minimize errors and increase their chances of earning full marks on the exam.
Understanding Chemical Equilibria in AP Exam
One of the fundamental concepts tested in the advanced exam is the understanding of dynamic balance in chemical reactions. Chemical equilibrium occurs when the forward and reverse reactions happen at the same rate, meaning the concentrations of reactants and products remain constant over time. Grasping this concept is essential for solving related problems, where you are often required to determine the direction of a reaction, calculate equilibrium constants, or predict the effects of various changes to the system.
In these types of questions, it is crucial to understand how different factors, such as temperature, pressure, and concentration, can shift the equilibrium position, as described by Le Chatelier’s principle. You will be expected to apply these principles to solve problems involving equilibrium constants and reaction quotients, making it important to both recall key formulas and demonstrate logical reasoning in your responses.
Mastering Stoichiometry for AP Exam
Stoichiometry is a vital skill for solving many problems in the advanced test. It involves using relationships between reactants and products in a chemical reaction to calculate quantities such as mass, volume, or moles. By mastering stoichiometric calculations, you can tackle a wide variety of questions, from determining the limiting reactant to calculating yields and concentrations. A solid understanding of this concept will help you solve problems more efficiently and with greater accuracy.
Key Stoichiometric Principles
The foundation of stoichiometry lies in the mole-to-mole ratios derived from balanced chemical equations. To successfully apply these ratios, it’s important to first ensure that the equation is balanced correctly. After that, conversions between grams, moles, and molecules can be performed using molar masses and Avogadro’s number. Practice with conversions and ratios is essential for fluency in stoichiometric calculations.
Common Stoichiometric Problems
Some typical problems in this area include determining the limiting reactant, calculating the theoretical yield, and finding percent yields. Each of these types of questions requires you to carefully follow each step and apply the correct conversions. Consistent practice with different problem types will build both speed and confidence, allowing you to approach these questions systematically during the exam.
Thermodynamics in AP Exam
Thermodynamics plays a crucial role in solving many questions on the advanced exam. It involves understanding the principles governing energy changes, particularly how energy is transferred during chemical reactions. Concepts such as enthalpy, entropy, and Gibbs free energy are frequently tested, with students expected to apply these principles to determine whether a reaction is spontaneous or to calculate the energy changes associated with specific reactions. A strong grasp of thermodynamic concepts is essential for tackling these types of problems effectively.
Key Thermodynamic Concepts
The core principles tested in the exam include the laws of thermodynamics, enthalpy changes, and the relationship between temperature and spontaneity. Here is a table summarizing the key formulas and concepts that are commonly tested:
| Concept | Formula | Explanation |
|---|---|---|
| Enthalpy Change | ΔH = H(products) – H(reactants) | Represents the heat absorbed or released in a reaction at constant pressure |
| Entropy Change | ΔS = S(products) – S(reactants) | Measures the disorder or randomness in a system |
| Gibbs Free Energy | ΔG = ΔH – TΔS | Indicates whether a reaction is spontaneous (ΔG |
Applying Thermodynamics to Solve Problems
When solving problems related to energy changes, it is essential to understand how these concepts interrelate. For example, a reaction may be exothermic but non-spontaneous if entropy decreases, while a reaction may be endothermic yet spontaneous if the increase in entropy is significant enough. Practice with calculations involving heat, entropy, and Gibbs free energy will help you answer related questions accurately and efficiently.
Redox Reactions Explained in Detail

Redox reactions, or oxidation-reduction reactions, are central to understanding many processes in science. These reactions involve the transfer of electrons between substances, leading to changes in their oxidation states. The substance that loses electrons is oxidized, while the one that gains electrons is reduced. Grasping the fundamentals of redox reactions is essential for solving problems related to electrochemistry, energy production, and various biochemical processes. Understanding how to identify these reactions and apply the right formulas is crucial for tackling questions involving this topic.
Identifying Oxidation and Reduction
In a redox reaction, it’s important to clearly identify which species is being oxidized and which is being reduced. The oxidation number of an element in a compound helps track electron transfer. When an element’s oxidation state increases, it has been oxidized, and when it decreases, it has been reduced. Here are the key steps to identifying oxidation and reduction:
- Assign oxidation states to all elements in the reactants and products.
- Identify the element that undergoes an increase in oxidation state (oxidation).
- Identify the element that undergoes a decrease in oxidation state (reduction).
Balancing Redox Reactions
Balancing redox reactions can be challenging, but it is essential for ensuring that the law of conservation of mass is upheld. This often involves balancing the electrons lost in oxidation with those gained in reduction. One popular method is the half-reaction method, where the reaction is split into two parts: one for oxidation and one for reduction. After balancing the two half-reactions, they can be combined to form the final balanced equation.
Key considerations in balancing redox reactions:
- Balance atoms involved in oxidation and reduction separately.
- Ensure the number of electrons gained equals the number of electrons lost.
- Account for changes in oxidation states and charge.
Acid-Base Reactions in Free Response
Acid-base reactions are a critical aspect of many problems encountered in the advanced exam. These reactions involve the transfer of protons between substances, leading to the formation of salts and the release or absorption of energy. A strong understanding of acid and base properties, along with the ability to apply equilibrium principles, is essential for solving related questions effectively. From calculating pH and pOH to understanding buffering systems, the ability to manipulate acid-base concepts is crucial for success in this section of the exam.
Key Concepts in Acid-Base Reactions
When dealing with acid-base reactions, it is important to understand key terms such as pH, pKa, and buffer solutions. The pH scale measures the acidity or basicity of a solution, with lower values indicating acidity and higher values indicating alkalinity. Additionally, the concept of strong and weak acids and bases plays a significant role in determining reaction outcomes and equilibrium positions.
Important acid-base principles:
- Strong acids and bases fully dissociate in solution, while weak acids and bases do not.
- Buffer systems help maintain a stable pH in solutions by neutralizing added acids or bases.
- pH can be calculated using concentration of hydrogen ions, and pOH is related to pH through the equation: pH + pOH = 14.
Solving Acid-Base Problems
To solve problems related to acid-base reactions, you must often calculate the concentration of hydrogen ions, determine the pH of a solution, or find the equilibrium concentrations in a buffer system. A step-by-step approach is crucial, starting with writing the balanced equation, identifying the concentrations of reactants and products, and then applying relevant formulas such as the equilibrium constant expression or the Henderson-Hasselbalch equation for buffer systems.
Practice with these calculations will help you approach problems more confidently and accurately during the exam.
Electrochemistry Insights from the 1999 Exam
Electrochemistry plays a pivotal role in understanding the relationship between electrical energy and chemical reactions. In the exam, questions on this subject test the ability to apply concepts such as oxidation-reduction reactions, galvanic cells, and electrolysis. A solid grasp of how to calculate cell potentials, identify half-reactions, and understand the principles of electrochemical cells is essential for addressing the challenges presented in this topic. Insights from past exams can provide valuable strategies for tackling complex electrochemical problems and achieving better results.
Key Concepts in Electrochemical Reactions
Electrochemical reactions involve the transfer of electrons between substances, typically through a conductor, leading to the generation of electrical energy. The two main types of electrochemical cells are galvanic (voltaic) cells, which produce electrical energy from spontaneous reactions, and electrolytic cells, which use electrical energy to drive non-spontaneous reactions. Understanding how to write half-reactions and calculate the overall cell potential is crucial when dealing with questions related to these cells.
- Galvanic cells convert chemical energy into electrical energy through spontaneous redox reactions.
- Electrolytic cells use electrical energy to drive non-spontaneous reactions, often in industrial applications like electroplating.
- Understanding the Nernst equation allows you to calculate the cell potential under non-standard conditions.
Common Questions in Electrochemistry
Electrochemical problems often require you to determine the cell potential, calculate the number of moles of electrons transferred, or predict the direction of electron flow. These problems may also include tasks such as writing half-reactions, balancing redox equations, or calculating the quantity of products formed during electrolysis. Familiarizing yourself with the standard electrode potentials and understanding how to use them in conjunction with the Nernst equation will be beneficial in answering such questions effectively.
- Calculating cell potential: Use the standard electrode potentials for both half-reactions to determine the overall potential.
- Balancing redox reactions: Ensure that the number of electrons lost in oxidation equals the number of electrons gained in reduction.
- Understanding how to relate the amount of electrical energy produced to the amount of substance involved in the reaction.
Mastering these key concepts and strategies will enable you to confidently tackle electrochemistry questions and apply your knowledge in various real-world contexts.
Practice with Kinetics Questions
Understanding the rate at which reactions occur and the factors influencing these rates is essential in many scientific disciplines. Questions in this area often require an in-depth analysis of reaction rates, rate laws, and how various parameters like concentration, temperature, and catalysts affect the speed of a reaction. To effectively address these types of questions, practice with problem-solving strategies and a thorough understanding of key concepts is crucial. By mastering these principles, you will be well-equipped to tackle kinetics-related queries.
Key Concepts in Reaction Rates
The rate of a reaction is influenced by several factors, including the concentration of reactants, temperature, and the presence of a catalyst. In most cases, a rate law equation expresses the relationship between the rate of reaction and the concentrations of reactants. Understanding the concept of reaction order and how to determine it experimentally is essential for solving problems in kinetics.
| Factor | Effect on Reaction Rate |
|---|---|
| Concentration | Increasing concentration typically increases reaction rate by providing more reactant particles to collide. |
| Temperature | Higher temperatures usually increase reaction rate due to more energetic collisions between molecules. |
| Catalyst | Catalysts lower activation energy and increase reaction rate without being consumed in the reaction. |
Types of Kinetics Questions
Common questions in this area involve determining the rate law from experimental data, calculating reaction rates at different conditions, or interpreting the effect of a catalyst on the rate of reaction. Additionally, you may be asked to calculate the activation energy using the Arrhenius equation or predict how changes in temperature will affect the rate constant. These questions test both conceptual understanding and mathematical problem-solving skills.
- Rate Law Determination: Analyze data to derive the rate law and the rate constant.
- Effect of Temperature: Use the Arrhenius equation to calculate changes in rate constants with temperature.
- Activation Energy: Solve for activation energy given the rate constants at different temperatures.
Regular practice with kinetics problems will enhance your ability to apply these concepts and solve related questions more efficiently. Focus on understanding the relationships between key factors and developing a systematic approach to problem-solving.
Preparing for Reaction Mechanism Problems
Understanding the step-by-step sequence of events in a chemical reaction is crucial for solving problems related to reaction mechanisms. These types of questions often require the identification of individual steps in the reaction, including the transition states, intermediates, and the overall reaction order. A strong grasp of these concepts helps in predicting how reactions proceed and how various factors influence their pathways.
Key Concepts in Reaction Mechanisms
Reaction mechanisms describe the detailed sequence of elementary steps that lead to the formation of products. Each step involves the breaking and making of chemical bonds, and the rate of each step is determined by factors such as concentration, temperature, and activation energy. Understanding how these elementary steps combine to form the overall reaction and how the rate law is related to the individual steps is essential for solving related problems.
Common Approaches for Solving Mechanism Problems
To solve reaction mechanism problems, focus on analyzing the provided data and recognizing the components of each elementary step. Start by identifying any intermediates, rate-determining steps, and how these factors relate to the overall reaction rate. Pay attention to reaction intermediates, catalysts, and how the mechanism fits with the observed rate law.
- Identify and write out the elementary steps involved in the reaction.
- Determine which step is the rate-determining step based on the reaction order.
- Match the rate law with the individual steps to predict the correct mechanism.
Practice with various mechanisms will help refine your ability to recognize patterns and apply principles like the conservation of mass, charge, and energy throughout each elementary step. It is also important to be familiar with common types of mechanisms, such as nucleophilic substitution or elimination reactions, as they often appear in practice problems.
Analysis of Molecular Structure Questions
Understanding the arrangement of atoms within a molecule is essential for solving questions related to molecular structures. These questions often focus on determining molecular geometry, bond angles, hybridization, and other structural characteristics based on the information provided. By analyzing how atoms are connected and how electrons are shared, students can predict various properties such as polarity, reactivity, and physical behavior.
Key Concepts in Molecular Structure
To accurately interpret molecular structure questions, it is important to grasp several key concepts:
- Bonding and Hybridization: The way atoms are connected and the type of orbitals they use for bonding can reveal a lot about molecular shape and properties.
- Molecular Geometry: This refers to the 3D arrangement of atoms in a molecule, determined by the repulsion between electron pairs.
- Electron Domains: Electron pairs surrounding the central atom determine the molecular geometry and bond angles.
Approaching Molecular Structure Questions
When faced with questions about molecular structure, the following steps can help you analyze the problem effectively:
- Count Valence Electrons: Determine the total number of electrons in the molecule, which will help you understand bonding and lone pairs.
- Determine Electron Domain Geometry: Identify the number of bonding and lone electron pairs around the central atom.
- Assign Hybridization: Based on the electron domain count, deduce the appropriate hybridization (sp, sp2, sp3, etc.).
- Predict Molecular Shape: Use the VSEPR theory to determine the 3D shape of the molecule.
- Analyze Bond Angles: Consider the geometry and hybridization to estimate the bond angles.
By applying these strategies, you can accurately address questions about molecular structure and make predictions about a molecule’s physical and chemical properties. Practice with different types of molecules will help reinforce these concepts and improve your ability to analyze complex structures.
Tips for Effective Time Management
Managing your time efficiently during an exam is crucial for success. With limited time and multiple tasks to complete, it is essential to have a strategy in place. Prioritizing tasks, pacing yourself, and staying organized can make a significant difference in your performance. By implementing effective time management techniques, you can ensure that you tackle every section of the exam with focus and clarity.
Strategies to Maximize Time Efficiency
Here are several proven strategies to manage your time more effectively during a timed exam:
- Prioritize Questions: Start by reading through all the questions quickly. Identify the ones you feel most confident about and answer them first. This will help you gain momentum and secure easy points.
- Time Allocation: Assign specific time limits to each section or question. For example, allocate 10 minutes per short-answer question and stick to it. This prevents you from spending too much time on one part of the exam.
- Stay Focused: Avoid distractions during the test. Stay focused on the task at hand and resist the temptation to dwell on difficult questions. If you’re stuck, move on and come back later with a fresh perspective.
- Practice Under Timed Conditions: Familiarize yourself with the timing of the exam by practicing under similar conditions. This helps build your pacing skills and prepares you for the pressure of the actual test.
Avoid Common Pitfalls
While time management is crucial, there are also common mistakes that can hinder your efficiency:
- Spending Too Much Time on One Question: Don’t spend excessive time on a question you’re struggling with. Move on and come back to it later if you have time remaining.
- Over-Analyzing: Sometimes, the simplest answer is the best one. Don’t overthink questions or second-guess yourself too much, as this can waste valuable time.
- Neglecting to Review: Always leave a few minutes at the end to review your answers. This ensures you don’t miss any mistakes or overlooked details.
By following these strategies and avoiding common time management errors, you can boost your performance and reduce stress during exams. With practice, managing time effectively becomes second nature, allowing you to focus on demonstrating your knowledge confidently.
Resources for Further Exam Preparation

When preparing for any challenging exam, it’s important to use a variety of study materials and resources to solidify your understanding of key concepts. Whether you’re seeking practice questions, instructional videos, or detailed textbooks, having access to diverse resources can help reinforce learning and boost confidence. Here are some valuable tools and approaches to enhance your preparation and maximize your chances of success.
Top Study Resources
Explore the following resources to deepen your understanding and ensure thorough preparation:
- Official Exam Guides: The official preparation materials, such as exam handbooks and sample questions, provide insight into the format and the types of tasks you’ll encounter. These resources are essential for familiarizing yourself with the exam structure.
- Textbooks: Detailed textbooks focused on relevant topics provide a solid theoretical foundation. They often include practice problems and worked-out examples that clarify complex concepts.
- Online Study Platforms: Websites and apps that offer interactive quizzes, video tutorials, and study notes are great for reinforcing knowledge. Platforms like Khan Academy, Coursera, and others offer free content tailored to exam preparation.
- Study Groups: Collaborative learning through study groups can help you better understand difficult topics. Discussing and explaining concepts to others reinforces your own knowledge.
Practice and Review Tools
In addition to the above, practicing with sample materials and reviewing previous exam papers is key to improving your performance. Consider the following:
- Past Exam Papers: Reviewing previous years’ test questions allows you to familiarize yourself with recurring themes, question types, and areas of focus. This practice helps you develop effective strategies for time management and answering.
- Practice Tests: Simulating exam conditions by taking full-length practice tests helps you build stamina and reduces anxiety. It also enables you to identify areas that need further review.
- Video Tutorials: Platforms like YouTube offer free video explanations of complex topics, allowing you to watch and rewatch difficult sections until they are clear.
- Flashcards: Using digital or physical flashcards for quick reviews of key terms, formulas, and concepts can be an effective way to reinforce memory.
By leveraging these resources, you can enhance your preparation, gain a deeper understanding of the material, and ultimately improve your performance on the exam. Consistent practice, focused review, and using a variety of study aids will help you approach the test with confidence and readiness.