
When studying the behavior of different substances, it is crucial to explore how their molecules interact with one another. These interactions play a significant role in determining the physical properties of matter, such as boiling and melting points, solubility, and the ability to conduct electricity. Understanding how molecules attract or repel each other helps explain many of the phenomena we observe in chemistry and everyday life.
In this section, we will explore the various types of interactions that occur between molecules, each influencing the substance in unique ways. From the attraction between polar molecules to the temporary shifts in electron density that lead to weak forces, these interactions are fundamental to the structure and behavior of matter. A deeper understanding of these concepts allows for a better grasp of chemical reactions and material properties, enriching the learning experience for students and professionals alike.
Through practical examples and detailed explanations, we aim to clarify how these molecular interactions influence the properties of substances. Whether you’re looking to solve related problems or simply deepen your understanding of molecular behavior, this guide provides the necessary insights to enhance your knowledge and comprehension of the subject.
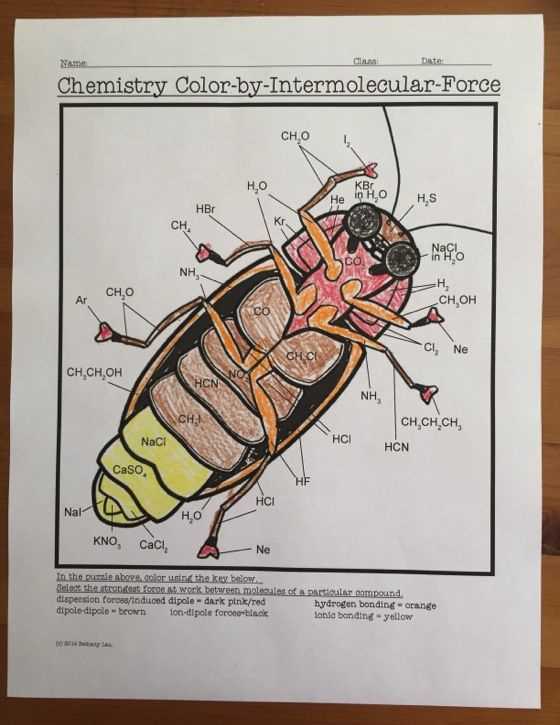
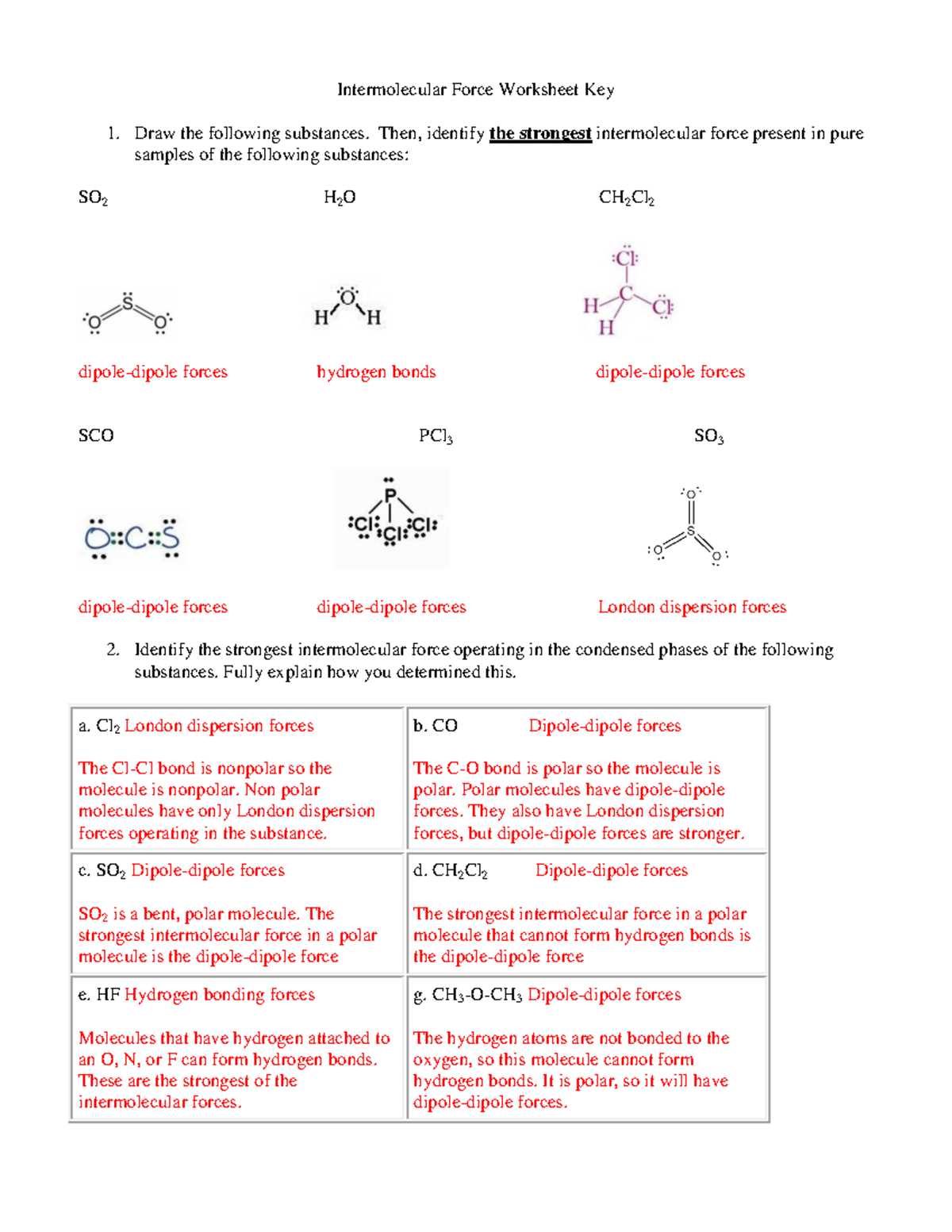
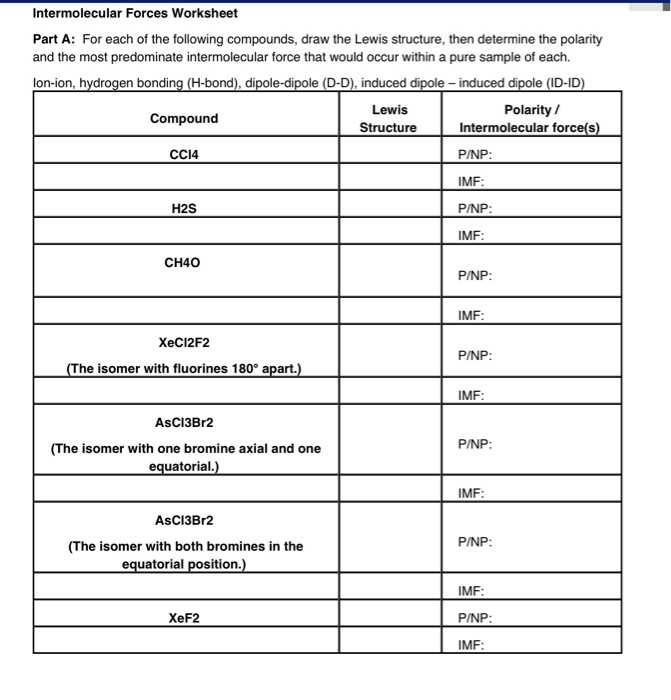
Intermolecular Forces Worksheet Answer Key

This section provides explanations and solutions to common problems related to the interactions between molecules. These interactions determine a wide range of physical properties and behaviors, such as how substances bond, their state at a given temperature, and how they respond to external conditions. Understanding the principles behind these interactions is essential for solving related exercises and grasping the core concepts of molecular chemistry.
The table below outlines typical problems and their solutions, helping you grasp the essential concepts more effectively. By reviewing these examples, you can better understand the impact of molecular behavior on substance characteristics and be more confident when tackling similar challenges in the future.
| Problem | Explanation | Solution |
|---|---|---|
| What type of interaction occurs in water molecules? | Water molecules exhibit a strong attraction due to polarity. | Hydrogen bonding |
| How do non-polar molecules interact? | Non-polar molecules experience temporary shifts in electron density, leading to weak attractions. | Dispersion forces |
| What happens to the boiling point when molecular interactions are stronger? | Stronger molecular interactions result in higher boiling points. | Increased boiling point |
| Which force is dominant in ionic compounds? | Ionic compounds exhibit strong attractions between charged particles. | Ion-ion interactions |
By studying these examples, you can begin to see how different types of molecular interactions govern the behavior of various substances. With this foundational knowledge, you’ll be better equipped to handle more complex questions and deepen your understanding of molecular chemistry.
Understanding Molecular Interactions
Molecular interactions are the key to understanding how substances behave under different conditions. These interactions determine many of the physical properties of materials, such as their solubility, boiling points, and even their ability to conduct electricity. By examining how molecules attract or repel each other, we gain insight into the nature of matter and the forces that shape chemical reactions and physical changes.
Types of Molecular Interactions
There are several types of molecular interactions, each with its own unique characteristics. These interactions occur at different strengths and have distinct effects on substances. The most common types include:
- Polar Interactions: Occur between molecules with permanent dipoles, where positive and negative charges attract.
- Hydrogen Bonds: A specific form of polarity where hydrogen atoms bond with electronegative atoms like oxygen or nitrogen.
- Dispersion Forces: Weak, short-range interactions that occur due to temporary shifts in electron density within molecules.
- Ionic Interactions: Strong attractions between positively and negatively charged ions.
Factors Affecting Molecular Behavior
Several factors influence how molecules interact with each other. Understanding these can help explain why some substances have higher boiling points, others dissolve easily in water, or why certain materials are better electrical conductors. The key factors include:
- Size and Shape: Larger molecules or those with complex structures often experience stronger interactions.
- Polarity: Polar molecules typically form stronger interactions compared to non-polar molecules.
- Temperature: Higher temperatures can disrupt molecular interactions, affecting the physical state of substances.
- Presence of Solvents: The surrounding medium can enhance or weaken molecular attractions, depending on the solvent’s properties.
By understanding these basic concepts, we can predict and explain the behavior of substances in various environments. This knowledge is fundamental for students, chemists, and anyone interested in molecular science.
Types of Molecular Interactions Explained
Molecules interact with each other in various ways, and understanding these interactions is key to explaining the physical properties of substances. The different types of attractions and repulsions between molecules influence everything from boiling points to the ability of a material to dissolve in a solvent. These interactions are fundamental to the behavior of matter and are classified based on the strength and nature of the attraction between molecules.
Polar Interactions
Polar molecules are those that have a permanent dipole, meaning they have a region with a partial positive charge and a region with a partial negative charge. This polarity leads to attractive interactions between molecules, with the positive end of one molecule attracting the negative end of another. These interactions are commonly seen in substances like water, where the dipoles form strong attractions that influence the liquid’s properties.
Hydrogen Bonds
Hydrogen bonding is a special case of polar interactions, but it is much stronger due to the specific nature of the atoms involved. In this type of interaction, a hydrogen atom, which is bonded to a highly electronegative atom (like oxygen or nitrogen), forms an attraction with another electronegative atom. These bonds are responsible for many of water’s unique properties, such as its high boiling point and surface tension.
Dispersion Forces
Dispersion forces, also known as London forces, occur in all molecules, whether polar or non-polar. These are weak, temporary interactions that happen when the electron distribution around a molecule becomes uneven, creating a temporary dipole. While individually weak, these interactions become more significant in larger molecules or in situations where many molecules are present.
Ionic Interactions
Ionic interactions occur between charged species, such as positively charged ions (cations) and negatively charged ions (anions). These interactions are much stronger than those seen in polar or dispersion forces because the attraction between opposite charges is significant. Ionic bonds are typically found in salts, where positively charged metal ions are attracted to negatively charged non-metal ions, creating a stable crystalline structure.
By understanding these different types of molecular interactions, we gain a clearer picture of why substances behave the way they do under various conditions. Each type of interaction plays a unique role in shaping the properties of matter, from its state at a given temperature to its solubility and reactivity.
How Hydrogen Bonds Affect Molecules
Hydrogen bonding plays a crucial role in shaping the behavior of many molecules, particularly those that contain highly electronegative atoms like oxygen, nitrogen, or fluorine. These interactions occur when a hydrogen atom, bonded to one electronegative atom, is attracted to another electronegative atom nearby. This type of bond is weaker than covalent bonds but still strong enough to significantly influence the physical properties of substances.
One of the most well-known examples of hydrogen bonding is found in water molecules. The oxygen atom in each water molecule is highly electronegative and attracts the hydrogen atoms from neighboring molecules. This leads to the formation of hydrogen bonds, which are responsible for water’s unique characteristics, such as its high boiling point, surface tension, and its ability to dissolve many ionic and polar substances.
Hydrogen bonds also have a significant effect on the structure and function of biological molecules like DNA and proteins. In DNA, hydrogen bonds hold together the complementary strands, allowing the molecule to maintain its double-helix structure. In proteins, these bonds help stabilize their three-dimensional shape, which is critical for their biological activity. Without hydrogen bonding, the complexity and function of these molecules would be drastically different.
In addition to their biological importance, hydrogen bonds influence the behavior of many organic compounds. For example, alcohols and acids, which contain hydrogen atoms bonded to electronegative atoms, exhibit properties such as higher boiling points and greater solubility in water due to hydrogen bonding. These interactions also affect the viscosity and volatility of various liquids.
Overall, hydrogen bonding is essential for understanding how molecules interact, how substances behave in different states, and how complex structures like DNA and proteins maintain their stability and function.
Van der Waals Forces and Their Role
Van der Waals interactions are weak attractions that occur between molecules or atoms due to temporary shifts in electron distribution. Though they are much weaker than other types of molecular attractions, they play a significant role in the behavior of substances, particularly in gases and non-polar compounds. These interactions can influence the physical properties of materials such as boiling points, melting points, and solubility.
There are two main types of Van der Waals interactions: London dispersion forces and dipole-induced dipole interactions. Each of these interactions contributes in different ways depending on the nature of the molecules involved.
Types of Van der Waals Interactions

- London Dispersion Forces: These are the most common and occur in all molecules, whether polar or non-polar. They arise due to fluctuations in electron density, which create temporary dipoles that induce similar dipoles in nearby molecules. Although these interactions are weak, they become more significant in larger molecules with more electrons.
- Dipole-Induced Dipole Interactions: These occur when a polar molecule induces a dipole in a non-polar molecule. The partial positive or negative charges in the polar molecule can distort the electron cloud of the nearby non-polar molecule, leading to a weak attraction between the two.
Role in Molecular Behavior
While Van der Waals interactions are weaker than ionic or hydrogen bonds, they still significantly affect the properties of many substances, especially gases and liquids. Some key influences include:
- Influence on Boiling and Melting Points: Substances with stronger Van der Waals interactions tend to have higher boiling and melting points, as more energy is required to overcome these attractions.
- Impact on Solubility: Molecules that experience stronger Van der Waals interactions are often more soluble in similar solvents, as they can interact more readily with solvent molecules.
- Effect on Molecular Structure: These interactions help stabilize the shapes of larger molecules, such as in proteins and polymers, by providing additional forces that hold the molecules together.
In summary, although Van der Waals interactions are relatively weak compared to other types of molecular attractions, they are crucial for understanding how molecules interact in gases, liquids, and non-polar environments. These forces help explain why certain materials behave the way they do and are essential for a comprehensive understanding of molecular chemistry.
The Importance of Dipole-Dipole Interactions
Dipole-dipole interactions play a vital role in determining the physical properties of many substances. These interactions occur between molecules that have a permanent dipole, where one part of the molecule is slightly positive and another part is slightly negative. The attraction between these oppositely charged regions of adjacent molecules can significantly affect a substance’s behavior, from its boiling point to its solubility and state of matter at room temperature.
Influence on Physical Properties
The strength of dipole-dipole interactions is directly related to the polarity of the molecules involved. Substances with stronger dipoles tend to exhibit more pronounced interactions, which can increase their boiling and melting points. For example, molecules such as hydrogen chloride (HCl) experience dipole-dipole attractions, which are stronger than the dispersion forces found in non-polar molecules, leading to higher boiling points compared to similar-sized non-polar molecules.
Impact on Molecular Behavior
These interactions also affect other important aspects of molecular behavior, including:
- Solubility: Polar molecules are more likely to dissolve in polar solvents due to the dipole-dipole interactions between them, making them miscible in substances like water.
- Stability of Molecules: Dipole-dipole interactions contribute to the overall stability of molecular structures, particularly in liquids and solids, where the alignment of positive and negative charges can help maintain molecular cohesion.
- Reactivity: The presence of dipole-dipole interactions can influence the reactivity of molecules by affecting their ability to form new bonds or react with other substances.
In summary, dipole-dipole interactions are a key factor in understanding how polar molecules behave in different environments. These interactions not only influence the physical properties of substances but also play a critical role in the chemical processes that occur in nature and in industrial applications.
London Dispersion Forces and Their Impact
London dispersion interactions, often referred to as temporary dipole-induced forces, are present in all molecules, though they are most noticeable in non-polar substances. These interactions arise due to momentary fluctuations in electron distribution, which create transient dipoles that induce similar dipoles in neighboring molecules. While individually weak, their cumulative effect can significantly influence the physical properties of a substance, especially in larger molecules or in certain states of matter.
How London Dispersion Interactions Work
Even in non-polar molecules, where there is no permanent dipole, the electrons are constantly in motion. At any given moment, the distribution of electrons may become uneven, creating a temporary dipole. This temporary dipole then induces a dipole in a nearby molecule, leading to a weak attraction between the two. Although these attractions are fleeting and very weak, they become stronger in larger molecules, which have more electrons and a greater likelihood of temporary dipoles.
Impact on Physical Properties
- Boiling and Melting Points: Molecules that are larger or have more electrons experience stronger London dispersion interactions, resulting in higher boiling and melting points compared to smaller molecules. For example, heavier noble gases like xenon and krypton, which experience stronger dispersion forces, have higher boiling points than lighter gases like helium or neon.
- Solubility: London dispersion interactions can affect the solubility of substances in non-polar solvents. Non-polar molecules, such as those in oils or fats, are more likely to dissolve in other non-polar substances because of the weak attractions between molecules.
- Molecular Stability: In larger molecules, the cumulative effect of London dispersion interactions can help stabilize their structure. This is particularly important in polymers and long-chain hydrocarbons, where these interactions contribute to the material’s overall physical strength and cohesiveness.
While these interactions may seem minor compared to other types of molecular attractions, they are essential in understanding how even non-polar molecules behave. In substances where other types of interactions are weak or absent, London dispersion interactions can have a significant impact on properties like phase transitions, stability, and reactivity.
Comparing Molecular Forces in Solids and Liquids
The arrangement and behavior of molecules differ significantly between solids and liquids, largely due to the varying strengths and types of interactions that hold them together. In solids, molecules are tightly packed and maintain a fixed position, while in liquids, molecules are more loosely associated and can move past one another. These differences in molecular behavior are influenced by the nature of the interactions between molecules in each state of matter.
Molecular Behavior in Solids
In solids, the molecules are held together by strong interactions that keep them in a rigid structure. The molecules vibrate but do not move freely, which is why solids have a definite shape and volume. The stronger the molecular attractions, the more rigid the structure will be. Some key characteristics include:
- Fixed Positions: Molecules are in a fixed, orderly arrangement, leading to a defined shape and volume.
- Limited Movement: Molecules can only vibrate in place due to the strong attraction between them, resulting in low molecular mobility.
- Higher Density: The tightly packed arrangement of molecules leads to high density in most solids.
Molecular Behavior in Liquids
In liquids, the molecular arrangement is more relaxed compared to solids. The molecules are still attracted to each other, but the interactions are weaker, allowing them to move past one another. This gives liquids the ability to flow and take the shape of their container. Important features include:
- Free Movement: Molecules can move around one another, allowing liquids to flow and change shape.
- Definite Volume, Indefinite Shape: Liquids maintain a constant volume but do not have a fixed shape, taking the shape of their container.
- Moderate Density: While the density is lower than that of solids, liquids are still denser than gases, due to closer molecular packing.
In both solids and liquids, the strength of molecular interactions plays a major role in determining the substance’s physical properties. In solids, the stronger interactions result in greater rigidity, while in liquids, weaker interactions allow for more flexibility and mobility between molecules. Understanding these differences is key to understanding how various substances behave in different states of matter.
How Molecular Interactions Influence Boiling Points
The boiling point of a substance is the temperature at which it transitions from liquid to gas. This transition occurs when the molecules in the liquid gain enough energy to overcome the attractions holding them together. The strength and nature of these attractions have a direct impact on how much energy is required for the molecules to break free and vaporize, thus influencing the boiling point. Molecules with stronger interactions generally require more energy to separate, leading to higher boiling points.
The more significant the molecular interactions, the greater the force that must be overcome for boiling to occur. For example, substances with strong attractions, such as hydrogen bonding or dipole interactions, typically have higher boiling points than those with weaker attractions, such as van der Waals forces. As a result, the boiling points of liquids vary widely depending on the type of molecular bonding present.
Impact of Stronger Interactions
When molecules are held together by strong attractions, more heat energy is needed to break these bonds and allow the substance to boil. Some factors include:
- Hydrogen Bonds: Molecules involved in hydrogen bonding, like water, have higher boiling points due to the strong interactions between hydrogen and electronegative atoms like oxygen.
- Dipole-Dipole Interactions: Polar molecules, such as hydrogen chloride, exhibit moderate boiling points because of the attractions between the positive and negative ends of neighboring molecules.
Effect of Weaker Interactions
In contrast, molecules held together by weaker interactions, such as London dispersion forces, require less energy to overcome. This results in lower boiling points, even if the molecules are larger in size. For example:
- Non-polar Molecules: Molecules like methane or helium, which experience only weak dispersion forces, have low boiling points because these interactions can be easily disrupted by adding heat.
- Smaller Molecules: Smaller non-polar molecules typically have lower boiling points due to fewer electrons and weaker temporary dipoles.
Understanding the relationship between molecular interactions and boiling points is essential for predicting how substances will behave under different conditions. Whether it is for industrial applications or scientific experiments, knowing how to manipulate these interactions can help control and refine processes involving liquids and gases.
Intermolecular Forces in Different States of Matter
The behavior of matter varies significantly across its different states: solid, liquid, and gas. The key to understanding these changes lies in the strength and nature of the attractions between molecules. In solids, molecules are closely packed together and held in place by strong interactions. In liquids, these interactions are weaker, allowing molecules to move past one another. In gases, the attractions between molecules are minimal, enabling them to move freely and spread out. The strength of these molecular attractions determines the physical properties of each state, including their shape, volume, and compressibility.
| State | Characteristics | Strength of Molecular Interactions |
|---|---|---|
| Solid | Definite shape and volume; molecules vibrate in fixed positions. | Strong interactions that keep molecules tightly packed. |
| Liquid | Definite volume but no definite shape; molecules can move past each other. | Moderate interactions that allow mobility but still maintain structure. |
| Gas | No definite shape or volume; molecules move freely and are widely spaced. | Weak or negligible interactions, allowing molecules to move independently. |
These differences in molecular behavior are influenced by the type of attraction between molecules, which varies in strength depending on the substance. For instance, in solids, strong molecular bonds create rigid structures, while in liquids and gases, weaker attractions lead to greater molecular freedom. Understanding how molecular interactions change across states helps explain the properties of various materials, from their melting and boiling points to their compressibility and density.
The Relationship Between Molecular Size and Forces
The size of a molecule plays a significant role in determining the strength of the interactions between its molecules. As the size of a molecule increases, so does the number of electrons and the overall surface area available for interactions. Larger molecules typically experience stronger attractive forces because they have more points of contact between their surfaces, leading to greater polarizability. This, in turn, affects the physical properties of substances, such as their boiling and melting points, as well as their solubility in different solvents.
Impact of Larger Molecules
When molecules are larger, they tend to have more electrons, which increases the strength of their temporary dipoles. This leads to stronger attractions between neighboring molecules. As a result, larger molecules typically have higher boiling and melting points. For example:
- Long-Chain Hydrocarbons: These molecules, such as those found in oils and waxes, exhibit stronger attractions compared to smaller molecules due to their larger size, resulting in higher melting points.
- Polymers: Large polymer molecules, which consist of repeated smaller units, also experience stronger interactions because of their size, leading to increased stiffness and higher structural integrity.
Effect of Smaller Molecules

On the other hand, smaller molecules, with fewer electrons and a smaller surface area, generally exhibit weaker interactions. These molecules tend to have lower boiling and melting points because their weak attractions can be easily disrupted by external energy. For example:
- Small Gaseous Molecules: Molecules like oxygen or nitrogen, which are small and non-polar, have weak attractions, resulting in low boiling points and the ability to exist as gases at room temperature.
- Low-Molecular-Weight Liquids: Smaller molecules in liquids, such as acetone, have weaker molecular interactions, which allows them to evaporate quickly and exhibit lower boiling points.
In summary, the relationship between molecular size and the strength of molecular interactions is a key factor in determining the physical properties of substances. Larger molecules generally experience stronger interactions, while smaller molecules exhibit weaker interactions, impacting their behavior in various environments.
Impact of Molecular Polarity on Forces
The polarity of a molecule plays a crucial role in determining the nature and strength of the interactions between its molecules. When a molecule has a positive and negative end, known as a dipole, it can interact with other molecules that also possess a dipole. These interactions are generally stronger than those found in non-polar molecules, where the electrons are more evenly distributed. The polarity of a molecule influences its physical properties, including solubility, boiling points, and melting points, as it determines how molecules will interact with each other and with other substances.
Polar Molecules and Their Interactions
In molecules with distinct positive and negative regions, the opposite charges attract, creating stronger interactions between molecules. This leads to higher melting and boiling points compared to non-polar molecules of similar size. Examples of polar molecules include:
- Water (H2O): Water is a highly polar molecule, and its strong dipole-dipole interactions lead to high cohesion between its molecules, resulting in its relatively high boiling point and ability to dissolve many substances.
- Ammonia (NH3): Another polar molecule, ammonia, experiences strong dipole-dipole interactions, which contribute to its unique properties like its relatively high boiling point compared to non-polar molecules of similar size.
Non-Polar Molecules and Their Interactions

In non-polar molecules, the distribution of electrons is symmetrical, leading to weaker interactions. These molecules do not have permanent dipoles but can develop temporary dipoles through momentary shifts in electron density. While these interactions are weaker, they still influence the behavior of the molecules. Non-polar substances are often characterized by:
- Low Boiling Points: Due to the lack of strong molecular interactions, non-polar molecules tend to have low boiling and melting points. For example, methane (CH4) is a non-polar molecule with a low boiling point.
- Solubility in Non-Polar Solvents: Non-polar molecules tend to dissolve in non-polar solvents due to similar types of weak molecular interactions. For instance, oil, a non-polar substance, dissolves in hexane, another non-polar solvent.
Overall, the polarity of a molecule significantly impacts its physical and chemical properties, affecting its interactions with other molecules, its behavior in different environments, and its ability to form mixtures or solutions with other substances.
Exploring the Role of Ion-Dipole Interactions
Ion-dipole interactions occur when charged particles, such as ions, interact with polar molecules. This type of interaction is particularly important in solutions, where ionic compounds dissolve in polar solvents, such as salts in water. These interactions are essential for many chemical processes, including the dissolution of salts, the conductivity of electrolytes, and the behavior of ions in biological systems. The strength of ion-dipole interactions depends on both the charge of the ion and the polarity of the molecule it interacts with.
How Ion-Dipole Interactions Work

In an ion-dipole interaction, the positive or negative charge of an ion is attracted to the partial charges in a polar molecule. For example, when sodium chloride (NaCl) dissolves in water, the positively charged sodium ions (Na+) are attracted to the partial negative charge on the oxygen atom of water molecules, while the negatively charged chloride ions (Cl–) are attracted to the partial positive charges of the hydrogen atoms in water.
- Example: In an aqueous solution of NaCl, the ion-dipole interactions help break the ionic bonds in NaCl, allowing it to dissociate into individual ions in solution.
- Strength of Interactions: The magnitude of these interactions is influenced by the charge of the ion and the dielectric constant of the solvent. Higher charged ions typically form stronger interactions with polar molecules.
Importance in Biological Systems
Ion-dipole interactions also play a crucial role in many biological processes. For instance, the interaction between ions and water molecules is vital in maintaining cellular function, controlling osmotic pressure, and facilitating enzyme activity. Additionally, the interaction of metal ions with specific proteins and other molecules in cells is key for the proper functioning of various biochemical pathways.
- Enzyme Activity: Many enzymes rely on ion-dipole interactions to function properly. The presence of ions helps stabilize enzyme-substrate complexes, enabling effective catalysis.
- Cell Signaling: Ion-dipole interactions are also involved in the transmission of signals across cell membranes, particularly in nerve cells where ion channels allow for the movement of ions in and out of cells.
Overall, ion-dipole interactions are fundamental in a wide range of chemical and biological phenomena, playing an essential role in dissolving substances, conducting electricity, and regulating cellular processes.
Key Differences Between Intermolecular and Intramolecular Forces
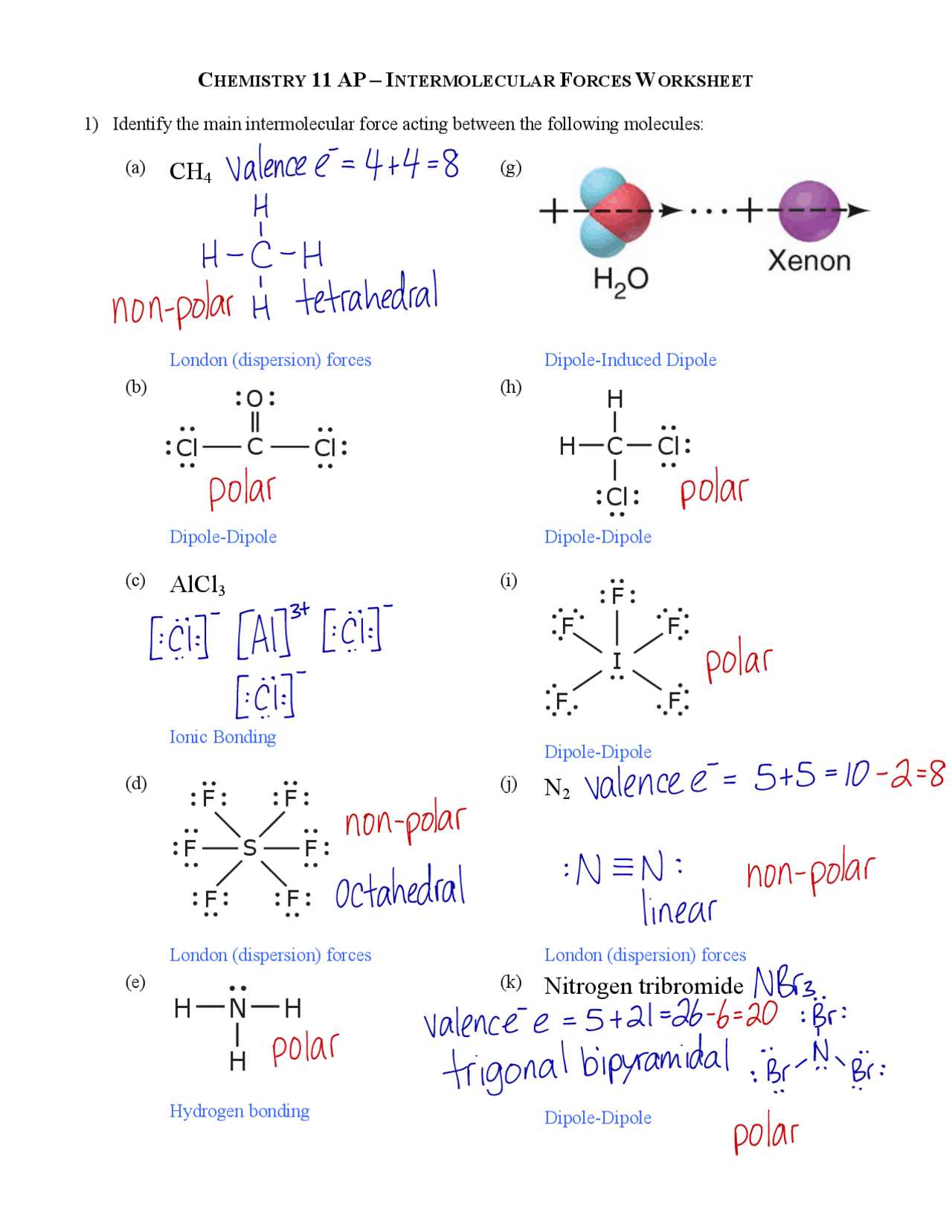
In the study of chemical bonding, it is essential to differentiate between the attractive interactions that occur within a molecule and those that occur between separate molecules. These two types of interactions, though similar in nature, play distinct roles in determining the properties and behavior of substances. While both are driven by electrostatic attraction, they differ in strength, influence on physical properties, and the way they govern molecular interactions.
Intramolecular Interactions

Intramolecular interactions occur within a single molecule and hold its atoms together. These interactions are responsible for the chemical bonding between atoms, including covalent bonds, ionic bonds, and metallic bonds. The strength of intramolecular interactions is typically much stronger than that of interactions between different molecules. These bonds determine the structure and stability of a molecule, influencing its chemical reactivity and the type of reactions it can undergo.
- Covalent Bonds: Atoms share electrons to form a stable bond. This bond is strong and holds the molecule’s atoms together.
- Ionic Bonds: Oppositely charged ions attract each other, forming a bond with high stability.
Interactions Between Separate Molecules
On the other hand, interactions between different molecules, such as Van der Waals forces, hydrogen bonds, and dipole-dipole interactions, are much weaker compared to intramolecular bonding. These forces influence how molecules interact with one another, affecting properties like boiling points, melting points, and solubility. While they do not determine the chemical structure of a molecule, they play a crucial role in determining the physical state of matter and the behavior of substances in different environments.
- Weaker Attractions: These interactions are weaker than intramolecular bonds and are often temporary.
- Impact on Physical Properties: These forces influence physical properties such as viscosity, surface tension, and boiling points.
In summary, intramolecular interactions are stronger and responsible for holding atoms together within a molecule, while interactions between molecules govern the physical properties of substances and how they interact in bulk. Understanding the distinction between these two types of interactions is key to explaining many phenomena in chemistry and material science.
Real-World Examples of Molecular Interactions
The interactions between molecules are not just theoretical concepts but play a critical role in everyday life and the functioning of various natural and industrial processes. These attractions between molecules influence a wide range of phenomena, from the behavior of liquids and gases to biological processes and material properties. Understanding these interactions helps explain many common occurrences in nature and technology.
One notable example is the way water behaves. The attraction between water molecules gives rise to surface tension, which allows small insects like water striders to “walk” on water without sinking. This is due to the cohesive forces between water molecules, which create a “film” on the water’s surface. Similarly, water’s relatively high boiling point compared to other molecules of similar size is also a result of these molecular attractions, requiring more energy to break the bonds between molecules.
In biology, molecular interactions are essential for the function of enzymes and proteins. The way enzymes bind to their substrates involves specific molecular attractions, which are crucial for the speed and efficiency of biochemical reactions. This is an example of how these interactions are integral to life processes, such as digestion and metabolism.
Another example can be found in the formation of gases and liquids. For instance, the condensation of water vapor into liquid droplets occurs when molecular attractions pull individual molecules together, turning the gas back into a liquid as temperature decreases. Similarly, when a substance changes from solid to liquid, molecular interactions must be overcome for the molecules to move freely, which is why different substances have different melting and freezing points.
In the world of materials science, understanding how molecules interact allows for the development of stronger, lighter, or more flexible materials. For example, the strength of plastics and polymers often comes from the ability of molecules to “stick” to each other in a way that gives the material its durability. In contrast, the flexibility of rubber is due to weaker molecular attractions, which allow the material to stretch and return to its original shape.
In summary, molecular interactions are central to a vast array of processes in the natural world and human-made technologies. From the way water behaves to the function of biological systems and the development of materials, these interactions help shape the world around us in many ways.
Tips for Solving Molecular Interaction Problems
Understanding the nature of molecular interactions can be challenging, but with the right approach, solving related problems becomes easier. When tasked with analyzing how molecules interact, it’s essential to break down the problem step by step, focusing on the key concepts that determine how molecules attract or repel each other. Here are some helpful tips to guide you through these types of questions effectively.
1. Identify the Type of Interaction: The first step in solving any problem related to molecular interactions is to identify the type of interaction involved. Determine whether you are dealing with attractions between polar molecules, nonpolar molecules, or ions. Understanding whether the molecules are polar or nonpolar will help you predict the strength and nature of the interactions.
2. Consider Molecular Structure: Pay close attention to the structure of the molecules. The arrangement of atoms and the presence of functional groups like hydroxyl or amine groups can significantly influence the type of interactions. A molecule’s shape and size also determine how closely it can approach other molecules, impacting the strength of the interaction.
3. Evaluate the Relative Strength of Interactions: Once you’ve identified the interaction type, it’s important to consider the relative strength of the interactions. Some interactions, like hydrogen bonding, are stronger than others, such as van der Waals forces. Knowing the strength helps in predicting physical properties like boiling and melting points, solubility, and vapor pressure.
4. Focus on Temperature and Pressure: The temperature and pressure conditions can affect how molecules interact with each other. At higher temperatures, molecules move faster, which can weaken interactions. In contrast, at lower temperatures, the movement slows down, allowing interactions to become stronger. Understanding how environmental conditions affect interactions is crucial for solving these problems accurately.
5. Draw Diagrams: Visualizing molecular interactions can sometimes make the concepts clearer. Drawing diagrams that show the orientation of molecules and the direction of their interactions can help you better understand the forces at play. This is particularly useful for understanding more complex interactions, such as dipole-dipole or ion-dipole attractions.
6. Practice with Examples: The best way to master problems involving molecular interactions is through practice. Work through multiple examples, and pay attention to the patterns in how different molecules interact. The more problems you solve, the more intuitive these concepts will become.
By following these tips and practicing regularly, you can improve your understanding and ability to solve problems related to molecular interactions. Remember, breaking down the problem, focusing on key factors, and applying your knowledge systematically will help you succeed.