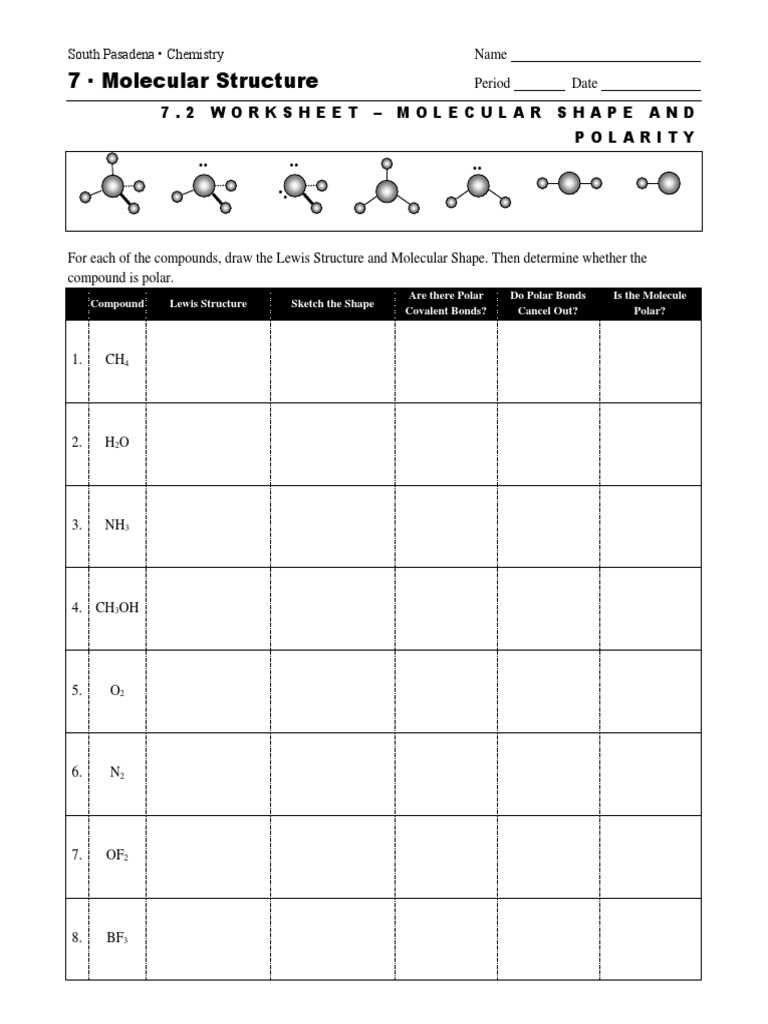
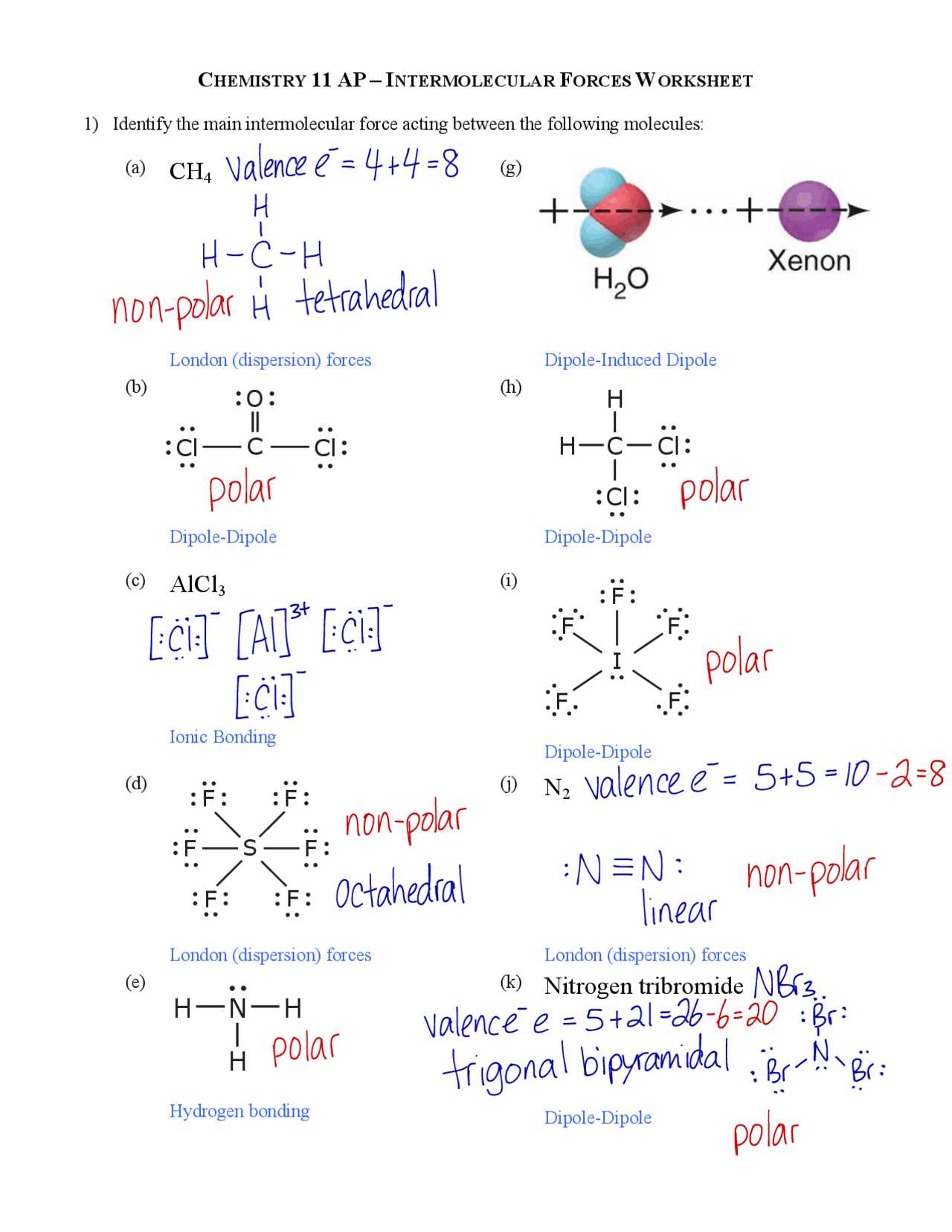
The study of how atoms are arranged within a molecule and how these molecules interact with one another is fundamental to understanding chemistry. The spatial arrangement of atoms in a molecule plays a crucial role in its physical and chemical properties, including its behavior in different environments.
By examining the various types of bonds and interactions between molecules, we can better predict how substances will react, dissolve, or form solids under certain conditions. These concepts are essential not only in theoretical chemistry but also in real-world applications like drug design, material science, and environmental studies.
In this section, we explore the key principles that govern molecular structure and the interactions between particles. We will focus on the most common types of bonding and how these influence the overall characteristics of different compounds. Understanding these principles is a critical step in mastering chemistry and its various practical applications.
Molecular Geometry and Intermolecular Forces Worksheet Answers
Understanding how particles are arranged in space and how they interact with each other is key to grasping fundamental principles in chemistry. These concepts allow us to predict the behavior of substances under various conditions and to understand their chemical reactivity. By exploring the relationships between structure and interaction, we can better analyze different materials and their properties.
The arrangement of atoms within a compound dictates its shape, which in turn influences its physical properties such as boiling point, melting point, and solubility. Additionally, the strength and type of interactions between molecules play a critical role in determining how substances behave in different environments.
| Concept | Description | Example |
|---|---|---|
| Bonding Types | The various connections between atoms that form molecules, including covalent, ionic, and metallic bonds. | Water (H2O) – covalent bonding |
| Electron Pair Repulsion | A model explaining how electron pairs arrange themselves to minimize repulsion, determining the shape of the molecule. | Ammonia (NH3) – trigonal pyramidal shape |
| Dipole Interactions | Attractive forces between molecules with partial positive and negative charges. | Hydrogen chloride (HCl) – dipole-dipole forces |
| Dispersion Forces | Weak interactions arising from temporary shifts in electron distribution, influencing nonpolar molecules. | Oxygen (O2) – London dispersion forces |
This section aims to provide a deeper understanding of how these concepts come together, highlighting both theoretical and practical applications. By reviewing typical examples and identifying key principles, students can reinforce their comprehension and apply it effectively in various chemistry-related challenges.
Understanding Molecular Geometry Concepts
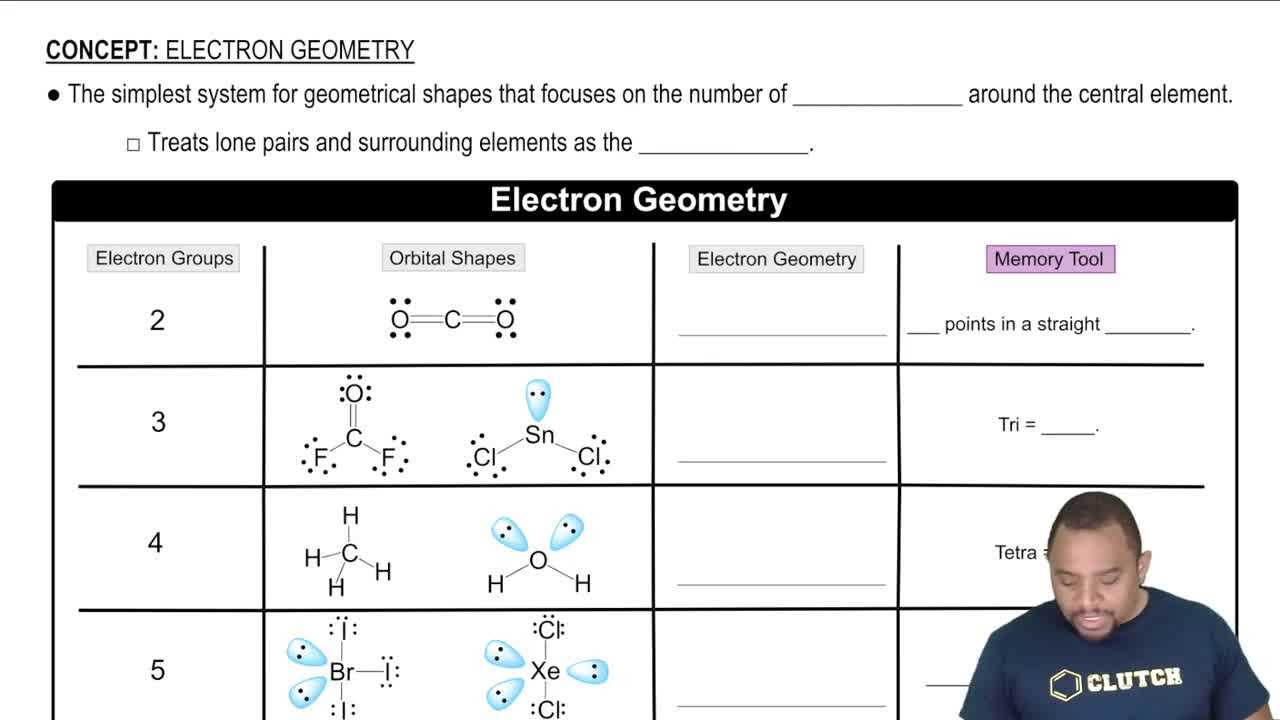
The arrangement of atoms within a substance plays a crucial role in determining its characteristics and behavior. The way atoms are positioned relative to one another affects the compound’s physical properties, such as shape, size, and polarity. A deeper understanding of these arrangements helps in predicting how substances will interact with each other and how they will behave in different environments.
Key Factors Affecting Structure
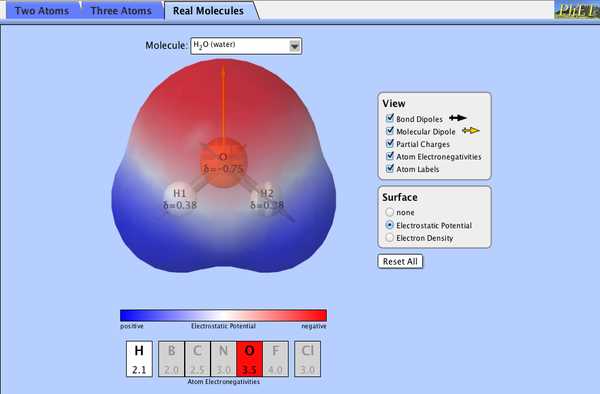
Several key factors influence the overall shape of a compound, including:
- Electron Repulsion: Electrons tend to repel each other, which impacts the spatial arrangement of atoms.
- Bond Angles: The angles between atoms determine the overall shape of the molecule and its behavior.
- Electron Clouds: The distribution of electrons in a molecule affects its shape and symmetry.
Common Molecular Shapes
Molecules can take on various shapes depending on how atoms are arranged. Some of the most common shapes include:
- Tetrahedral: A central atom is surrounded by four other atoms in a pyramid-like shape.
- Linear: Atoms are arranged in a straight line.
- Trigonal Planar: Three atoms are positioned around a central atom, forming a flat triangle.
- Trigonal Pyramidal: A three-atom arrangement with one atom above the plane of the other three.
Understanding these basic concepts is essential for predicting how molecules will behave in chemical reactions and how they interact with one another. By grasping the principles behind atomic arrangements, students can apply this knowledge to solve more complex chemical problems.
Types of Chemical Bonds and Structures
The way atoms are connected within a compound is fundamental to understanding its properties and behavior. The nature of these connections determines how substances interact, their stability, and their reactivity. There are different types of connections between atoms, each with its own distinct characteristics and influence on the overall structure of a compound.
Types of Chemical Bonds
There are several types of connections that can form between atoms, including:
- Covalent Bonds: Atoms share pairs of electrons, creating a strong bond between them. This is common in nonmetallic compounds.
- Ionic Bonds: Atoms transfer electrons, resulting in charged particles (ions) that are held together by electrostatic attraction.
- Metallic Bonds: Electrons are shared freely between many atoms, creating a “sea of electrons” that gives metals their unique properties, such as conductivity and malleability.
Structural Considerations
The way atoms are arranged in a substance also affects its overall structure. Common structural arrangements include:
- Simple Cubic: Atoms are arranged in a repeating cube pattern, where each atom is equidistant from its neighbors.
- Hexagonal Close-Packed: Atoms are arranged in a hexagonal pattern, maximizing the packing efficiency.
- Face-Centered Cubic: Atoms are arranged in a way that each corner of the cube has an atom, along with additional atoms at the center of each face.
Understanding these bonding types and structural arrangements is essential for predicting how compounds will behave in chemical reactions and their physical properties. The different bonds and structures provide insight into why substances exhibit certain behaviors, such as conductivity, solubility, and melting points.
The Role of Electron Pairs in Geometry
The arrangement of atoms within a compound is significantly influenced by the presence and behavior of electron pairs. These pairs, whether bonding or nonbonding, play a key role in determining the overall shape of a substance. The repulsion between these electron pairs causes them to spread out in a way that minimizes conflict, which in turn affects the spatial configuration of the atoms in the structure.
Electrons within a molecule occupy regions of space that are called electron clouds. Bonding electrons are shared between atoms, while lone pairs belong to a single atom. The interaction between these electron clouds governs how atoms are positioned relative to one another. This behavior is critical for understanding the physical properties of compounds, such as their boiling points, polarity, and reactivity.
The electron repulsion model helps explain how different atoms arrange themselves in space, whether they form straight lines, pyramidal shapes, or more complex structures. By examining these patterns, it becomes easier to predict how molecules will behave in various chemical reactions and how they will interact with other substances.
Factors Influencing Molecular Shape
The shape of a compound is determined by several factors, all of which contribute to how atoms are arranged within the substance. These factors can significantly impact the physical and chemical properties of a substance, including its reactivity, polarity, and stability. Understanding the forces at play can help predict how a compound will behave under different conditions.
Key Factors Affecting Shape
The arrangement of atoms within a molecule depends on the following primary factors:
- Electron Pair Repulsion: Electron pairs around a central atom push each other away, causing atoms to arrange themselves in a way that minimizes these repulsive forces.
- Bonding Types: The type of bond (single, double, or triple) can influence how atoms are spaced and the overall shape of the molecule.
- Lone Pair Electrons: Nonbonding electrons also affect shape, as they take up space and cause repulsion with bonding electron pairs.
- Atom Size: The size of the atoms involved in bonding can influence the distances between them, affecting the overall shape of the compound.
Influence of Electron Clouds
The electron cloud surrounding each atom plays a key role in determining the geometry of a compound. Electron pairs tend to spread out as much as possible to reduce repulsion, leading to specific shapes based on how the atoms are arranged.
| Factor | Effect on Shape |
|---|---|
| Electron Pair Repulsion | Pushes atoms to positions that minimize repulsion, affecting overall structure. |
| Bonding Types | Affects the angles between atoms and overall molecular configuration. |
| Lone Pair Electrons | Take up space and influence the positioning of bonded atoms, causing distortion. |
| Atom Size | Determines the spacing between atoms, influencing overall molecular shape. |
These factors interact in complex ways, determining the final shape of a substance. A clear understanding of these influences helps chemists predict how molecules will behave in different environments, including their reactions with other substances.
VSEPR Theory Explained Simply
One of the most important concepts in understanding the shape of a compound is the idea that electron pairs around an atom repel each other. This theory helps explain how atoms in a molecule are arranged in space to minimize this repulsion. By focusing on how atoms and electrons interact, we can predict the three-dimensional shape of substances and understand their physical properties more clearly.
Basic Principles of the Theory
The theory suggests that electron pairs surrounding a central atom will arrange themselves in a way that minimizes the repulsive forces between them. This leads to specific shapes depending on the number of electron pairs, both bonding and nonbonding, around the central atom.
- Electron Pairs: Electrons tend to spread out in the available space to reduce repulsion.
- Bond Angles: The distance between bonded atoms is influenced by how far apart the electron pairs are, which creates specific bond angles.
- Electron Clouds: The electron clouds around each atom push against one another, guiding the spatial arrangement of atoms.
Common Shapes Predicted by the Theory

By applying the principles of this theory, several common shapes can be predicted, depending on the number of electron pairs around the central atom. These shapes help determine the overall properties of a substance:
- Linear: Atoms are arranged in a straight line with a bond angle of 180°.
- Tetrahedral: Four bonds are arranged symmetrically around the central atom, creating a 109.5° bond angle.
- Trigonal Planar: Three bonds are arranged flat in a triangle, creating 120° bond angles.
- Trigonal Pyramidal: Three bonds form a pyramid shape, with one lone pair affecting the structure.
By understanding the basic ideas of this theory, we can better predict how molecules will behave and react in different environments, based on their shape and the distribution of electrons.
Understanding Intermolecular Forces Overview
Substances are held together by various types of interactions between their particles. These interactions influence many properties, such as boiling points, solubility, and vapor pressure. Understanding these interactions is essential for predicting how materials will behave in different conditions. These forces occur between molecules or atoms and are critical in determining how compounds interact with each other and with their environment.
Types of Interactions
There are several key types of interactions that influence the behavior of substances. Each type of interaction has different strengths and properties:
- Dispersion Forces: Weak, temporary attractions caused by momentary shifts in electron density within molecules.
- Dipole-Dipole Interactions: Occur between polar molecules, where positive and negative ends attract each other.
- Hydrogen Bonds: A strong type of dipole-dipole interaction that occurs when hydrogen is bonded to electronegative elements like oxygen, nitrogen, or fluorine.
- Ionic Interactions: Form between oppositely charged ions, typically found in ionic compounds.
Influence of These Interactions on Properties
The strength of these interactions plays a crucial role in determining the physical properties of substances, such as their boiling and melting points, viscosity, and surface tension. Understanding these interactions helps explain why certain substances are more volatile than others, why some are soluble in water, and why others form strong bonds at room temperature.
| Interaction Type | Effect on Properties |
|---|---|
| Dispersion Forces | Usually weak but can become significant in large molecules, affecting boiling points. |
| Dipole-Dipole | Stronger than dispersion, causing polar substances to have higher melting and boiling points. |
| Hydrogen Bonds | Lead to high boiling points, solubility in water, and are crucial for life processes. |
| Ionic Interactions | Lead to very strong bonds, resulting in high melting and boiling points in salts. |
These interactions govern the structure and behavior of compounds, which in turn determines their practical applications in various fields, from industrial processes to biological systems.
Dipole-Dipole Interactions and Their Impact
When molecules have a permanent distribution of charge, they experience a specific type of attraction between their positive and negative regions. These attractions, occurring between molecules with partial positive and negative charges, significantly affect various physical properties. The strength of these interactions determines how substances behave, including their boiling points, solubility, and reactivity.
Mechanism of Attraction
In compounds with polar characteristics, the positive end of one molecule attracts the negative end of another. This attraction occurs because the electrons in the molecule are unevenly distributed, creating a dipole. The result is a continuous pull between molecules, where like charges repel and opposite charges attract, leading to a stable arrangement that minimizes energy.
- Polar Molecules: Molecules with a significant difference in electronegativity between bonded atoms tend to form permanent dipoles, making them capable of dipole-dipole interactions.
- Orientation Effect: The direction in which molecules align also affects the strength of the interaction, with more favorable orientations resulting in stronger attractions.
- Impact on Physical Properties: Dipole-dipole interactions tend to increase melting and boiling points compared to nonpolar substances of similar size, as the additional attractive forces require more energy to overcome.
Influence on Substance Behavior
These interactions play a key role in determining how molecules arrange themselves in different environments. For example, polar molecules are more likely to dissolve in polar solvents due to the compatibility of their charges, while nonpolar molecules interact differently. This understanding helps explain why some liquids mix easily while others do not, and why certain compounds are more stable in specific conditions.
Furthermore, dipole-dipole attractions are crucial for understanding processes such as protein folding in biology, where the arrangement of charged regions within the protein structure influences its final shape and function.
London Dispersion Forces in Detail
Even in nonpolar molecules, there are subtle attractions that arise due to fluctuations in the distribution of electrons around atoms. These temporary imbalances create brief dipoles, which can influence other nearby particles. Although these interactions are weak compared to others, they are universal, present in all molecules, and play a crucial role in the behavior of many substances, particularly those that are larger or more complex.
Mechanism of Attraction
As electrons move within an atom or molecule, they can momentarily create a temporary dipole, where one region becomes slightly more negative and the other more positive. This fluctuation in electron distribution induces similar dipoles in neighboring molecules, leading to an attraction between them. These attractions are called London dispersion interactions and occur regardless of whether the molecule itself is polar or nonpolar.
- Temporary Dipoles: The fleeting electron shifts create temporary regions of positive and negative charges that attract one another.
- Induced Dipoles: The presence of one temporary dipole can influence neighboring molecules, inducing them to form their own temporary dipoles.
- Distance Dependency: These interactions become stronger as molecules are closer together, but rapidly decrease as the distance increases.
Impact on Physical Properties
While London dispersion forces are weak in comparison to other types of attractions, they can significantly influence the physical properties of substances, especially in larger molecules. As molecules increase in size, the number of electrons increases, strengthening the dispersion forces. This explains why larger nonpolar molecules, like certain gases and hydrocarbons, can exist as liquids or solids at lower temperatures than their smaller counterparts.
- Boiling and Melting Points: Substances with stronger dispersion interactions often have higher boiling and melting points compared to smaller molecules.
- Viscosity: London dispersion forces contribute to a substance’s resistance to flow, which increases as these interactions become stronger.
- Solubility: Larger nonpolar substances tend to dissolve in each other due to these interactions, even if no permanent dipole exists in the molecules.
Overall, London dispersion interactions are an essential part of understanding the behavior of substances, especially in gases and organic compounds, where they contribute to many phenomena such as condensation and phase transitions.
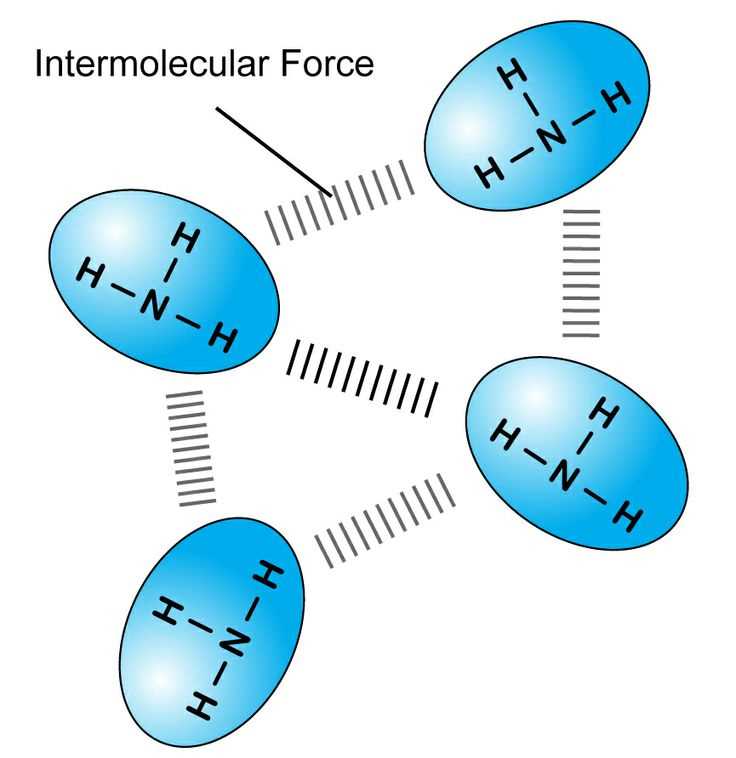
Hydrogen Bonding in Molecular Interactions
Among the various types of attractions between molecules, one of the strongest and most significant involves a specific type of interaction between hydrogen and highly electronegative atoms like oxygen, nitrogen, or fluorine. This interaction plays a critical role in determining the properties and behaviors of many substances, influencing everything from water’s high boiling point to the structure of DNA. It arises when a hydrogen atom, covalently bonded to a highly electronegative atom, forms a bond with another electronegative atom nearby.
How Hydrogen Bonding Works

In substances where hydrogen is attached to an electronegative atom, the bond creates a partial positive charge on the hydrogen and a partial negative charge on the electronegative atom. This polarization allows the hydrogen atom to interact with a lone pair of electrons on another electronegative atom in a nearby molecule. These interactions are stronger than regular dipole-dipole forces but are still weaker than covalent or ionic bonds.
- Electronegativity: The strength of hydrogen bonding depends heavily on the electronegativity of the atoms involved. Oxygen, nitrogen, and fluorine are the most common elements involved in these interactions.
- Distance: Hydrogen bonds are highly dependent on the distance between molecules. The closer the molecules, the stronger the attraction.
- Orientation: The relative orientation of the interacting molecules also plays a role in the strength of the bond, with optimal alignment leading to stronger interactions.
Applications and Implications
Hydrogen bonding has significant implications for both biological systems and industrial processes. In water, these bonds are responsible for many of its unique properties, such as its high specific heat, surface tension, and solvent capabilities. Hydrogen bonding also plays a central role in the formation of the secondary and tertiary structures of proteins, as well as the helical structure of DNA.
Additionally, this type of bonding is essential in many chemical reactions and processes. For example, it impacts the solubility of various compounds in water and influences the behavior of polymers, affecting their flexibility and strength.
| Property | Effect of Hydrogen Bonding |
|---|---|
| Boiling Point | Increases due to the extra energy needed to break hydrogen bonds |
| Water Solubility | Enhances solubility of polar compounds in water |
| Protein Structure | Stabilizes the structure by maintaining specific shapes |
| Viscosity | Increases viscosity due to the network of hydrogen bonds |
Overall, hydrogen bonding is a crucial aspect of many chemical and biological systems, providing unique properties that are vital to life and various industrial applications.
Ionic vs Covalent Bonding Differences
When atoms combine to form compounds, they do so by forming bonds. The nature of these bonds–whether ionic or covalent–determines many of the compound’s properties, including its strength, conductivity, and melting point. The key difference between these types of bonds lies in how the atoms involved share or transfer electrons, leading to distinct characteristics in the resulting substances.
Ionic Bonding
In ionic bonding, one atom donates an electron to another, resulting in the formation of oppositely charged ions. The electrostatic attraction between these ions holds them together. This type of bond typically occurs between elements with a large difference in electronegativity, such as metals and nonmetals. The resulting compounds often form solid crystalline structures and tend to have high melting points, as the ionic bonds are strong and require a significant amount of energy to break.
- Electron Transfer: One atom loses electrons, becoming positively charged, while the other gains electrons, becoming negatively charged.
- Properties: Ionic compounds usually have high melting and boiling points and are often soluble in water.
- Conductivity: Ionic compounds conduct electricity when dissolved in water or melted, as the ions are free to move.
Covalent Bonding
Covalent bonding involves the sharing of electrons between atoms. This type of bond typically occurs between nonmetals, where atoms share electrons to achieve a full outer electron shell, often resulting in more stable compounds. Covalent bonds can be either polar or nonpolar, depending on how equally or unequally the electrons are shared between the atoms involved. These compounds tend to have lower melting points than ionic compounds and may exist as gases, liquids, or solids at room temperature.
- Electron Sharing: Atoms share one or more pairs of electrons to achieve a stable configuration.
- Properties: Covalent compounds tend to have lower melting points and are not typically conductive.
- Conductivity: Most covalent compounds do not conduct electricity, as they do not have free ions or electrons.
In summary, the differences between ionic and covalent bonding are significant. Ionic bonds result from electron transfer and typically lead to strong, high-melting compounds, while covalent bonds involve electron sharing, leading to substances that may vary widely in their properties depending on the nature of the atoms involved.
Molecular Geometry and Polarity Relationships
The shape of a compound plays a crucial role in determining its overall behavior, especially when it comes to how the molecule interacts with other substances. The distribution of charge across a molecule, or its polarity, is closely linked to its structure. Understanding the relationship between the arrangement of atoms and the distribution of electrons is essential to predict a molecule’s physical properties such as solubility, boiling point, and reactivity.
Understanding Polarity in Molecules
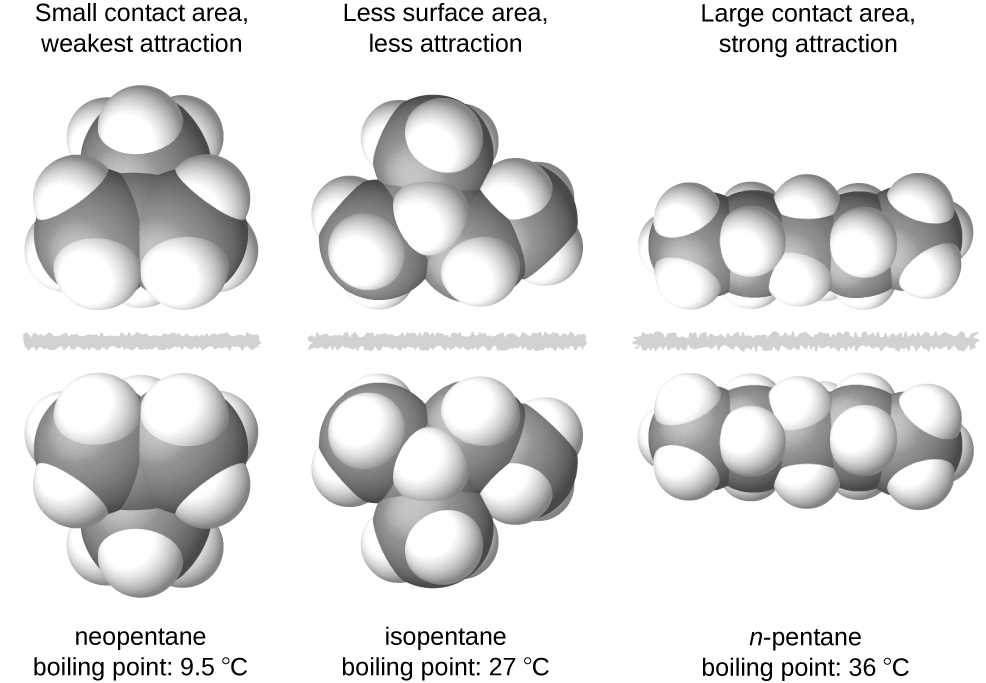
Polarity in a compound arises due to the uneven sharing of electrons between atoms, often caused by differences in electronegativity. This leads to a dipole moment, where one part of the molecule becomes partially negative, and another part becomes partially positive. The shape of the molecule influences whether these dipoles cancel each other out or combine to create a net dipole moment.
- Electronegativity Differences: When atoms with different electronegativities bond, the electrons are drawn more towards the more electronegative atom, creating a partial charge.
- Dipole Moment: A molecule with a net dipole moment has a positive and a negative side, resulting in overall polarity.
- Impact of Shape: The three-dimensional arrangement of atoms affects how the dipoles align, influencing the polarity of the whole molecule.
Impact of Shape on Polarity
The overall shape of a molecule plays a key role in determining its polarity. Even if a molecule contains polar bonds, the symmetry of its structure can cause the dipoles to cancel each other out, resulting in a nonpolar molecule. Conversely, an asymmetrical structure can lead to a net dipole, making the molecule polar. Here are some examples of how shape influences polarity:
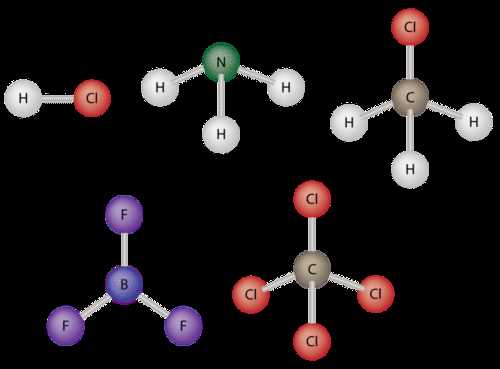
- Tetrahedral Shape: In a symmetrical tetrahedral structure, like in methane (CH₄), the dipoles cancel out, making it nonpolar.
- Linear Shape: A linear molecule, like carbon dioxide (CO₂), may have polar bonds, but due to its symmetry, the dipoles cancel each other, making the molecule nonpolar.
- Bent Shape: A bent molecule, like water (H₂O), has an asymmetrical distribution of electron density, resulting in a net dipole moment and making the molecule polar.
In conclusion, the shape of a molecule is a determining factor in whether it will exhibit polarity or not. While the individual bonds within a molecule may be polar, the overall geometry can result in the cancellation or reinforcement of these dipoles, thus shaping the molecule’s behavior in different environments.
Analyzing Molecular Geometry Using Models
Understanding the structure of a compound is essential for predicting its behavior and interactions with other substances. One effective way to explore these structures is through the use of models. Models allow us to visualize how atoms are arranged within a molecule, and how this arrangement influences its properties. By using different types of models, chemists can better understand the spatial relationships between atoms and how these relationships affect the molecule’s overall function.
There are several types of models used to represent molecular structures, each offering a unique perspective on how atoms are bonded and arranged. These models can help explain key concepts such as bond angles, the presence of lone pairs, and the overall shape of the molecule. Below are the main types of models used in molecular analysis:
- Ball-and-Stick Models: These models use spheres to represent atoms and sticks to represent bonds between them. They provide a clear, three-dimensional view of the molecule’s structure and bond angles.
- Space-Filling Models: Unlike ball-and-stick models, space-filling models show the relative sizes of atoms and how they fit together. They help visualize the molecule as a whole, showing the occupied space around each atom.
- Electron Cloud Models: These models focus on the distribution of electrons around the atoms, giving insight into the electron density and how it affects the chemical behavior of the molecule.
By using these models, it becomes easier to identify key structural features such as the presence of symmetry, bond angles, and the arrangement of atoms in space. This visual representation is vital for understanding how molecules behave in different conditions, and how their structures contribute to properties like polarity, reactivity, and stability.
Ultimately, the use of models provides a simplified yet powerful tool for chemists to predict and explain the behavior of compounds, making the analysis of their structure more intuitive and accessible.
Common Errors in Geometry and Force Predictions
Predicting the structure and interactions of compounds can be challenging, especially when it comes to understanding the spatial arrangement of atoms and the resulting interactions between molecules. Even experienced chemists can make errors when analyzing these characteristics. These mistakes often arise from misinterpreting key factors or overlooking important variables that influence the overall behavior of the substance. Recognizing common errors can help avoid misconceptions and improve the accuracy of predictions.
One common error is failing to account for lone pairs of electrons when determining the shape of a molecule. These electron pairs, though not involved in bonding, still influence the molecular arrangement and can significantly affect bond angles and overall structure. Neglecting to include them in models can lead to incorrect predictions about a molecule’s properties.
Another frequent mistake is assuming that all bonds are equal in strength and length. In reality, bonds within a molecule can vary in strength depending on the type of atoms involved and the environment surrounding them. Over-simplifying the interactions between atoms can lead to inaccurate predictions about the molecule’s stability and reactivity.
In addition, failing to consider the impact of temperature and pressure on the behavior of molecules is another common error. These external factors can alter the forces between particles and change the way molecules interact with one another. Predicting molecular behavior without accounting for these variables can result in misleading conclusions about the substance’s properties under different conditions.
Lastly, misinterpreting the symmetry of a molecule can lead to errors in predicting its polarity. Even small variations in symmetry can influence the overall distribution of charge within a molecule, which in turn affects its behavior in electric fields and its interactions with other molecules.
By carefully considering these common pitfalls and being aware of the underlying factors that influence molecular behavior, more accurate predictions can be made, leading to a deeper understanding of chemical interactions.
Practical Applications of Molecular Geometry
The arrangement of atoms within a substance plays a crucial role in determining its physical properties, chemical reactivity, and overall behavior. Understanding how atoms are positioned in a molecule provides valuable insights into a wide range of applications, from material science to pharmacology. This knowledge is essential for designing new materials, drugs, and technologies that improve our daily lives.
Applications in Drug Design
In pharmaceuticals, the shape and structure of molecules directly affect their interaction with biological systems. Drug molecules are often designed to fit perfectly with specific receptors in the body, similar to how a key fits into a lock. The arrangement of atoms in a compound can influence its ability to bind with proteins, enzymes, or other biological targets. By understanding how atoms are positioned in a drug molecule, researchers can optimize its efficacy and reduce potential side effects.
Material Science and Nanotechnology
The properties of materials, including strength, flexibility, conductivity, and resistance to heat, are determined by the arrangement of atoms and the type of bonds formed between them. In nanotechnology, manipulating atomic and molecular structures at the nanoscale allows scientists to create new materials with unique properties. From electronics to lightweight materials, the ability to predict and control molecular structure is essential for innovation in these fields.
Influence on Physical Properties
Many physical properties of substances, such as boiling points, melting points, and solubility, are governed by the spatial arrangement of atoms and the interactions between molecules. For example, molecules with highly symmetrical structures tend to have lower boiling points due to weaker interactions between them, while irregular structures may exhibit stronger intermolecular forces, leading to higher boiling points. Understanding these relationships helps chemists predict how substances will behave under different conditions.
Environmental Impact and Sustainability
In environmental science, understanding the structure of pollutants and how they interact with other substances is vital for designing effective treatments and remediation strategies. Molecules with certain shapes and characteristics may be more easily broken down or neutralized in the environment. By studying these interactions, scientists can develop more sustainable practices and technologies to address environmental issues like pollution and climate change.
- Designing better pharmaceuticals for targeted therapies.
- Creating innovative materials for advanced technologies.
- Predicting the physical properties of substances for industrial applications.
- Developing sustainable solutions for environmental challenges.
In conclusion, the practical applications of understanding atomic arrangements are vast and diverse, impacting industries from healthcare to manufacturing. By continuing to study and manipulate these structures, scientists can continue to drive advancements that improve human health, technology, and the environment.
Intermolecular Forces in Solubility
The ability of a substance to dissolve in a solvent depends largely on the nature of the interactions between the molecules of the solute and solvent. When particles of a solute dissolve in a solvent, it’s the balance between these interactions that determines whether the process will occur and to what extent. The strength and type of these interactions play a pivotal role in solubility, affecting everything from the dissolution of salts in water to the mixing of oils and alcohols.
Types of Interactions in Solubility
There are several types of interactions that influence how well a substance dissolves in a given solvent. The compatibility between the solute and solvent molecules can lead to various outcomes. Some of the most common types include:
- Hydrogen Bonding: This occurs when hydrogen atoms attached to electronegative atoms like oxygen or nitrogen form bonds with lone electron pairs on another electronegative atom. Water, for example, can dissolve many polar substances due to its hydrogen-bonding ability.
- Van der Waals Forces: These are weak interactions that occur between all molecules, but are especially significant in nonpolar substances. They can influence solubility, particularly in organic compounds.
- Dipole-Dipole Interactions: Molecules with permanent dipoles interact with each other, aligning in such a way that the positive end of one molecule attracts the negative end of another. These interactions can make some polar compounds highly soluble in polar solvents.
- Ionic Interactions: Charged ions in ionic compounds can interact with polar solvents, especially those that can stabilize the charged particles, such as water. This is the mechanism behind the solubility of salts in aqueous solutions.
Factors Affecting Solubility
Several factors influence how these interactions work together to determine solubility:
- Temperature: Higher temperatures generally increase the solubility of solids in liquids, as it helps break up the solute-solute interactions and allows for more solute-solvent interactions to form.
- Pressure: For gases, an increase in pressure usually results in higher solubility. This is why carbonated beverages are stored under high pressure to keep carbon dioxide dissolved.
- Nature of the Solute and Solvent: “Like dissolves like” is a useful rule of thumb. Polar solutes tend to dissolve in polar solvents, while nonpolar solutes are more likely to dissolve in nonpolar solvents due to the similarity in their intermolecular interactions.
By understanding these interactions and factors, chemists can better predict and manipulate solubility in various applications, from pharmaceuticals to environmental science.
How Molecular Geometry Affects Reactivity
The structure and shape of a compound significantly influence its chemical reactivity. How atoms are arranged within a molecule affects how it interacts with other molecules or reagents. This arrangement can determine the strength of interactions, the ease with which bonds are broken or formed, and ultimately the speed and nature of chemical reactions. A molecule’s spatial configuration directly impacts how it behaves in a reaction, influencing factors such as accessibility to reactive sites and the overall stability of the compound.
Key Factors Influencing Reactivity
Several factors related to the structure of a compound play crucial roles in its reactivity:
- Bond Angles: The angles between bonds in a molecule determine how well it can align with other molecules during a reaction. For example, molecules with more rigid structures may find it harder to undergo certain reactions because they cannot easily align with reactants.
- Bond Strength: The strength of bonds within a molecule affects how easily they can be broken. Molecules with weaker bonds may react more readily, while those with stronger bonds may require more energy or specific conditions to react.
- Electron Density Distribution: The distribution of electrons across a molecule can influence reactivity by affecting how it interacts with electrophiles or nucleophiles. Molecules with high electron density in certain regions may be more prone to attacking positively charged species.
- Functional Groups: The presence of specific groups within a molecule, such as hydroxyl or carbonyl groups, can dramatically change how the molecule reacts. These functional groups are often the sites of reactivity in many chemical processes.
Impact of Shape on Reactivity
The three-dimensional shape of a molecule can also significantly influence how it reacts. Some molecules may have reactive centers that are shielded by bulky groups or atoms, making them less likely to interact with other compounds. Conversely, molecules with more exposed reactive sites are generally more reactive. For instance:
- Steric Effects: The size and shape of a molecule can hinder or enhance its ability to approach other molecules. Large substituents can block access to reactive sites, reducing the molecule’s reactivity.
- Shape Complementarity: Molecules that fit well with each other in terms of shape are more likely to react, as seen in enzyme-substrate interactions or the binding of drugs to receptors.
By understanding how shape influences reactivity, chemists can design compounds that react more efficiently or selectively, leading to more controlled chemical processes in fields like drug development, material science, and industrial chemistry.
Summary of Key Worksheet Answers
This section provides a concise overview of the essential concepts covered in the previous sections. It emphasizes the critical points that contribute to a deeper understanding of the structure and interactions of different compounds. Key topics include how atoms are arranged, how they influence reactivity, and the various types of interactions that play a role in the behavior of substances. By reviewing these main ideas, one can gain insights into predicting chemical properties and reactivity patterns.
Important Concepts
- Atomic Arrangement: The way atoms are positioned within a compound determines its overall behavior and its interactions with other molecules. The spatial distribution of electrons affects reactivity, accessibility to other molecules, and the strength of bonds.
- Reactivity Factors: Key elements such as bond angles, bond strength, and electron density distribution influence how a substance reacts. Understanding these aspects can guide the prediction of reaction mechanisms.
- Functional Groups: Specific groups within molecules often serve as the primary sites of reactivity. Recognizing these groups is crucial for understanding how compounds interact with other substances.
- Shape and Size: The overall shape and size of a molecule can significantly impact its interactions and reactivity. Steric effects can block or facilitate reactions depending on how a molecule is structured.
Key Takeaways

- Bonding Arrangements: The arrangement of bonds within a compound directly influences how it will behave in chemical processes. This includes both its reactivity and stability.
- Electron Distribution: The distribution of electrons throughout a molecule is essential in determining how it will interact with other substances, such as in reactions with electrophiles or nucleophiles.
- Functional Group Influence: Functional groups are central to chemical reactivity, often acting as the primary sites where reactions take place.
These key concepts serve as a foundation for understanding chemical behavior and can be applied to predict the properties and reactions of various substances. Whether in laboratory settings or industrial applications, grasping these fundamental ideas is crucial for anyone studying chemistry or working in related fields.