Understanding the principles behind various chemical processes is essential for success in any related subject. This section provides a comprehensive guide to mastering core concepts, which will help you navigate challenges and perform confidently in your studies. By breaking down complex theories and offering practical insights, we aim to make even the most intricate topics accessible.
From the fundamentals of reaction mechanisms to the application of laws governing electrochemical behavior, the key to mastering these topics lies in practice and understanding the underlying principles. Focusing on problem-solving strategies, we highlight the most common topics and methods used in assessments, providing a solid foundation for anyone looking to improve their knowledge.
Preparation involves more than just memorizing definitions–it requires the ability to apply knowledge in different contexts. Whether you are tackling practical exercises or theoretical challenges, mastering these critical concepts will significantly boost your confidence and performance.
Electrochemistry Exam Questions and Answers
Mastering key concepts and solving related problems is essential for success in understanding the behavior of chemical reactions in various conditions. This section provides a thorough exploration of the topics typically covered, guiding you through the process of addressing common challenges faced in assessments. We break down complex scenarios into manageable steps, focusing on both theoretical principles and practical problem-solving techniques.
Core Topics for Effective Study
To build a strong foundation, it’s crucial to focus on the major themes that appear regularly in academic tasks. These include the understanding of oxidation-reduction processes, calculating cell potentials, and applying laws such as Faraday’s. Each of these plays a fundamental role in solving practical problems and interpreting experimental results. Ensuring you are comfortable with these core ideas will make tackling any challenge more intuitive.
Approaching Problem-Solving
Once familiar with the theory, it’s time to hone your problem-solving skills. The best approach involves breaking each challenge down into smaller steps, focusing first on the given information and then applying the appropriate formulas. Practice with diverse examples helps you recognize patterns and improve your speed and accuracy. By consistently working through problems, you’ll gain the confidence needed to handle even the most complicated tasks with ease.
Key Concepts in Electrochemistry
Understanding the fundamental principles behind chemical reactions involving electron transfer is crucial for mastering the subject. These core concepts serve as the foundation for solving practical problems and interpreting experimental results. By grasping the essential topics, you can build a stronger understanding and improve your problem-solving skills.
- Redox Reactions: The study of oxidation and reduction processes, where one substance loses electrons while another gains them.
- Cell Potential: The ability to measure the energy change in a reaction and predict the spontaneity of a process.
- Faraday’s Laws: These laws relate to the relationship between the amount of substance deposited and the amount of charge passed during electrolysis.
- Electrode Potentials: Understanding the voltage associated with electrodes in different chemical environments, which helps determine the direction of electron flow.
- Electrolytic and Galvanic Cells: The two main types of cells where chemical energy is converted into electrical energy or vice versa.
By mastering these essential ideas, you can tackle complex problems with more confidence and clarity. With practice, these concepts become powerful tools for solving a variety of theoretical and practical challenges in the field.
Understanding Redox Reactions
Redox reactions are at the heart of many chemical processes, involving the transfer of electrons between substances. These reactions are essential for understanding energy changes and predicting the behavior of materials in different environments. A solid grasp of how these reactions work is crucial for solving related problems and interpreting experimental data.
- Oxidation: The process in which an atom, ion, or molecule loses electrons. This often results in an increase in the oxidation state of the substance.
- Reduction: The opposite process, where an atom, ion, or molecule gains electrons, leading to a decrease in its oxidation state.
- Electron Transfer: The fundamental action in redox reactions, where electrons move from the substance being oxidized to the substance being reduced.
- Oxidizing and Reducing Agents: The substances that facilitate oxidation and reduction. The oxidizing agent gains electrons, while the reducing agent loses electrons.
- Half-Reactions: Redox reactions are often split into two half-reactions: one showing oxidation and the other showing reduction, helping to visualize the electron transfer process.
Recognizing these key components allows for a deeper understanding of the behavior of substances during these reactions. Whether analyzing chemical reactions in a lab or predicting the outcomes of industrial processes, mastering redox is essential for accurate assessments and solutions.
Types of Electrochemical Cells
In chemical processes that involve the movement of electrons, different types of cells play a significant role in converting chemical energy into electrical energy or vice versa. These cells vary in structure and function, each serving specific purposes in various applications. Understanding the differences between them helps in selecting the right type for particular reactions and technologies.
Galvanic Cells

Galvanic cells, also known as voltaic cells, generate electrical energy through spontaneous reactions. These cells are commonly used in batteries and other power sources where chemical reactions drive the flow of electrons.
- Spontaneous Reactions: In these cells, the chemical reaction occurs without external energy input, producing electricity as a byproduct.
- Structure: They consist of two half-cells, each containing an electrode immersed in an electrolyte solution.
- Examples: Common examples include standard batteries like AA and lead-acid batteries.
Electrolytic Cells
Electrolytic cells, unlike galvanic cells, require an external power source to drive non-spontaneous reactions. These cells are used in industrial processes such as electroplating and the production of chemicals like chlorine and hydrogen.
- Non-Spontaneous Reactions: These cells require electrical energy to induce a reaction that would not occur naturally.
- Structure: Electrodes are placed in an electrolyte solution, and an external current is applied to drive the reaction.
- Examples: Electroplating, water splitting, and the extraction of metals are typical applications of electrolytic cells.
Both types of cells serve essential functions in various fields, from powering electronic devices to producing materials on an industrial scale. A solid understanding of their operation helps in optimizing their use in practical applications.
Galvanic and Electrolytic Cells

Cells that involve the movement of electrons are critical for various chemical reactions, with two primary types being central to many processes: those that generate electrical energy and those that require an external power source to drive reactions. These two cell types–galvanic and electrolytic–are distinct in their mechanisms, applications, and the direction of energy flow.
Galvanic cells are designed to produce electricity through spontaneous reactions. In these systems, a chemical reaction occurs naturally, releasing energy in the form of electrical current. They are commonly found in everyday devices such as batteries and fuel cells. These cells consist of two half-cells, each with its own electrode immersed in an electrolyte, where the energy from the reaction drives the flow of electrons through an external circuit.
On the other hand, electrolytic cells operate in the opposite direction, requiring an external power source to initiate and sustain chemical reactions. Unlike galvanic cells, where energy is released, electrolytic cells absorb electrical energy to carry out reactions that would not naturally occur. These cells are used in processes such as electroplating, metal extraction, and water splitting, where electrical current facilitates the breakdown of compounds into their constituent elements.
Both cell types play significant roles in industries ranging from power generation to materials production, with their applications driven by their ability to either generate or consume electrical energy. Understanding the differences between them is crucial for applying the right technology to specific needs.
Electrochemical Nernst Equation Explained
The Nernst equation is a fundamental tool used to calculate the equilibrium potential for a specific ion in a system. It provides insight into how the concentration of ions influences the voltage across an electrochemical cell. By applying this equation, you can determine the potential at which there is no net movement of ions across the membrane, allowing for better understanding of both spontaneous and non-spontaneous reactions.
This equation is especially useful when dealing with redox reactions and helps to predict how changes in ion concentration affect the overall cell potential. It allows for a quantitative relationship between ion concentration and the potential, which is vital for understanding processes such as cellular respiration, nerve signal transmission, and the behavior of batteries under different conditions.
Mathematically, the Nernst equation can be written as:
E = E0 – (RT/nF) * ln([ion outside]/[ion inside])
Where:
- E is the equilibrium potential for the ion.
- E0 is the standard electrode potential.
- R is the gas constant.
- T is the temperature in Kelvin.
- n is the number of electrons involved in the reaction.
- F is the Faraday constant.
- [ion outside] and [ion inside] represent the ion concentrations outside and inside the cell, respectively.
By plugging in the appropriate values for these variables, the Nernst equation can help predict how ions will move and the potential difference across a membrane, which is crucial in many biological and chemical systems.
Standard Electrode Potentials Overview

Standard electrode potentials are a crucial concept in understanding the relative strengths of different substances to gain or lose electrons in a redox reaction. They allow us to predict the direction of electron flow and determine the spontaneity of reactions. By measuring the potential of an electrode in a standard solution, we can quantify how easily a substance is reduced or oxidized compared to a standard reference electrode, typically the hydrogen electrode.
The standard electrode potential is expressed in volts (V) and represents the tendency of a half-reaction to occur under standard conditions (298 K, 1 M concentration for solutions, and 1 atm pressure for gases). A positive value indicates a strong tendency to gain electrons, while a negative value suggests a stronger tendency to lose electrons. These values are essential for calculating the overall cell potential and understanding the energy changes during reactions.
| Substance | Standard Electrode Potential (V) |
|---|---|
| Hydrogen (H2) | 0.00 |
| Copper (Cu2+/Cu) | +0.34 |
| Silver (Ag+/Ag) | +0.80 |
| Iron (Fe2+/Fe) | -0.44 |
| Zinc (Zn2+/Zn) | -0.76 |
By comparing the standard electrode potentials of different substances, we can predict which one will undergo reduction and which will undergo oxidation in a given redox reaction. The greater the difference between the electrode potentials, the more energy is released in the reaction. These values are fundamental for understanding galvanic cells, electrolytic processes, and many other applications in chemical and biological systems.
Applications of Electrochemistry in Industry
The principles of electron transfer and redox reactions are applied across various industrial processes, playing a key role in numerous fields. From energy production to material processing, these concepts help optimize efficiency, reduce costs, and create more sustainable practices. The applications range from energy storage systems to metal extraction, with each leveraging different aspects of chemical reactions to achieve specific goals.
Energy Storage and Batteries
One of the most prominent uses of these principles is in the design and operation of batteries and energy storage systems. By utilizing chemical reactions to store and release electrical energy, industries can develop efficient power sources for everything from mobile devices to electric vehicles. Rechargeable batteries, like lithium-ion and lead-acid, depend on reversible electrochemical reactions that allow for repeated charging and discharging cycles.
Metal Extraction and Electroplating

In the metal industry, these reactions are essential for extracting metals from ores and refining them. Electrolysis, for example, is commonly used to separate metals like aluminum and copper from their compounds. Additionally, electroplating techniques are used to coat objects with a thin layer of metal, providing benefits such as improved corrosion resistance and enhanced appearance. The precision and controllability of these processes are key advantages in manufacturing.
Overall, these industrial applications highlight the importance of controlling and understanding electron flow, enabling advancements in energy technologies, materials production, and environmental sustainability. The continual evolution of these methods promises further innovations that could impact a wide array of industries in the future.
Understanding Faraday’s Laws of Electrolysis
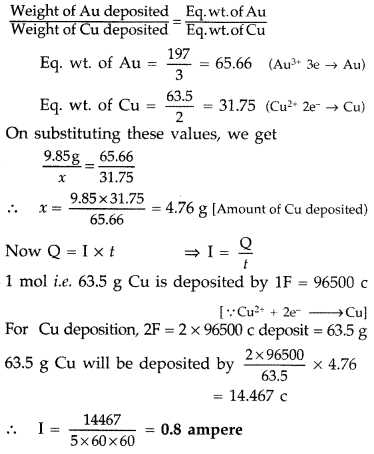
Faraday’s laws of electrolysis describe the relationship between the amount of substance transformed during an electrochemical reaction and the amount of electric charge passed through the system. These laws provide a foundational understanding of how electric current interacts with matter in processes such as electroplating, electrorefining, and the extraction of metals. By applying these laws, we can predict how much material will be deposited or dissolved at the electrodes based on the current and the duration of electrolysis.
First Law of Electrolysis
The first law states that the amount of substance deposited or dissolved at an electrode is directly proportional to the quantity of electric charge passed through the electrolyte. This means that the more charge that flows, the more material will be transformed at the electrodes. The equation for this relationship is:
m = k × Q
Where:
- m is the mass of the substance deposited or dissolved.
- k is a constant that depends on the substance.
- Q is the total charge passed, which is the product of the current and time (Q = I × t).
Second Law of Electrolysis
The second law provides that when the same amount of electric charge passes through different electrolytes, the mass of substances deposited at the electrodes is inversely proportional to their equivalent weights. This law allows us to compare the efficiency of different reactions under identical conditions, based on the chemical properties of the substances involved.
m₁/m₂ = E₂/E₁
Where:
- m₁ and m₂ are the masses of the substances deposited from the two electrolytes.
- E₁ and E₂ are the equivalent weights of the substances.
| Substance | Equivalent Weight (g/equiv) |
|---|---|
| Copper (Cu) | 31.8 |
| Silver (Ag) | 107.9 |
| Zinc (Zn) | 32.7 |
By using Faraday’s laws, we can calculate the precise amount of material needed for electroplating or extraction processes, which is crucial for industrial applications where precision and cost-effectiveness are key. These laws continue to serve as a core principle in modern electrochemical technologies.
How to Solve Electrochemical Problems
Solving problems in the field of redox reactions and ion flow requires a systematic approach, focusing on understanding the underlying principles of chemical reactions, electrode potentials, and charge transfer. By following a clear process, one can predict reaction outcomes, calculate cell potentials, and analyze the behavior of ions in various solutions. Here is a guide to approaching such problems effectively.
Step 1: Identify the Half-Reactions
The first step in solving any problem involving redox processes is to break down the overall reaction into two half-reactions: one for oxidation and one for reduction. Identifying which substance undergoes oxidation (losing electrons) and which undergoes reduction (gaining electrons) is essential for understanding the flow of electrons and predicting the direction of the reaction.
- Write out the oxidation and reduction half-reactions separately.
- Balance the atoms in each half-reaction, ensuring that both mass and charge are conserved.
- Include any spectator ions and adjust the equations accordingly.
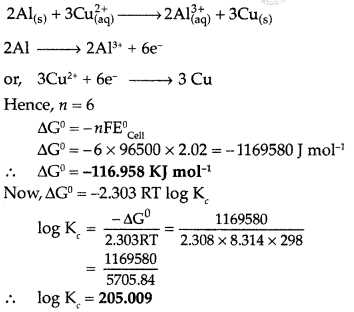
Step 2: Calculate the Cell Potential
Once you have the half-reactions, you can calculate the overall cell potential using standard electrode potentials. The cell potential will help you determine whether the reaction is spontaneous (positive value) or non-spontaneous (negative value).
- Find the standard electrode potentials for both half-reactions.
- Use the following formula to calculate the cell potential: Ecell = Ecathode – Eanode.
- If the result is positive, the reaction is spontaneous under standard conditions; if negative, the reaction is non-spontaneous.
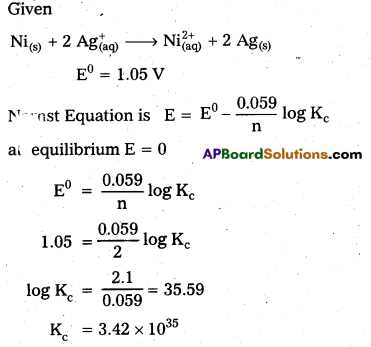
Step 3: Use Nernst Equation for Non-Standard Conditions
If the reaction conditions are not standard (i.e., concentrations of reactants and products are not 1 M), you can use the Nernst equation to calculate the actual cell potential. The equation helps account for the effect of concentration on the voltage of the electrochemical cell.
- The Nernst equation is: E = E0 – (RT/nF) * ln(Q)
- Where R is the universal gas constant, T is the temperature in Kelvin, n is the number of electrons transferred, F is Faraday’s constant, and Q is the reaction quotient.
- Substitute the known values into the equation to find the corrected cell potential.
By following these steps, you can systematically approach any redox reaction problem, whether it involves simple half-reactions or more complex systems requiring advanced calculations. Understanding the interplay of voltage, ion concentration, and reaction spontaneity is key to mastering these concepts and applying them to real-world scenarios.
Calculating Cell Potential in Reactions
In any electrochemical process, the cell potential plays a crucial role in determining whether a reaction is spontaneous or not. It represents the driving force behind the flow of electrons between the two electrodes. Calculating this potential involves understanding the relative tendencies of two half-reactions–reduction and oxidation. By knowing the electrode potentials of each half-reaction, one can easily determine the overall voltage of the cell and predict its behavior under different conditions.
The standard electrode potential, E0, is a key factor in this calculation. It indicates the ability of a half-cell to attract electrons compared to the standard hydrogen electrode (SHE), which is defined as 0 volts. To calculate the overall cell potential, you need to subtract the potential of the anode (oxidation site) from that of the cathode (reduction site).
The formula for calculating cell potential is:
Ecell = Ecathode – Eanode
Where:
- Ecathode is the reduction potential at the cathode.
- Eanode is the oxidation potential at the anode.
- Ecell is the overall cell potential.
If the resulting Ecell is positive, the reaction will proceed spontaneously under standard conditions. A negative Ecell indicates a non-spontaneous reaction. In cases where the conditions deviate from the standard state (i.e., concentrations are not 1 M), the Nernst equation can be applied to adjust for these differences and provide an accurate calculation of the potential.
By using these equations and understanding the significance of the cell potential, you can effectively analyze a wide range of reactions and their feasibility. Whether you are studying the process of electrolysis, metal extraction, or corrosion, mastering the calculation of cell potential is essential for predicting outcomes and optimizing reaction conditions.
Importance of Concentration Cells
Concentration cells are an essential concept in understanding how ion concentration differences can create electrical potential. These cells consist of two half-cells that are made from the same material but contain different concentrations of ions. The potential difference arises purely due to the variation in concentration, and the cell operates by the flow of ions from a region of higher concentration to a region of lower concentration. This process helps explain various natural and industrial processes that rely on concentration gradients.
How Concentration Cells Work
In a concentration cell, the electrochemical reaction is driven by the difference in ion concentration between the two half-cells. The half-cell with a higher concentration of ions will act as the anode (oxidation site), while the half-cell with the lower concentration will act as the cathode (reduction site). Electrons flow from the anode to the cathode through an external circuit, and ions move through the electrolyte to balance the concentrations. The result is the generation of an electrical potential, which can be measured using a voltmeter.
Applications and Significance

Concentration cells have wide-ranging applications. They play a critical role in processes like corrosion, where a metal surface with varying exposure to electrolytes can create a potential difference that accelerates degradation. In biological systems, concentration gradients across cell membranes drive crucial processes such as nutrient absorption and waste removal. Concentration cells are also used in sensors and batteries, where maintaining specific ion concentrations can influence performance and efficiency.
Understanding the importance of concentration cells is key for optimizing chemical reactions and developing technologies that depend on ion transport, such as fuel cells and desalination systems. The principles behind concentration cells allow for a deeper insight into how concentration differences can impact the flow of electrons and influence energy production and storage.
Electrochemical Methods of Analysis
Electrochemical analysis methods are widely used to investigate the composition and properties of substances by observing their behavior in electrical fields. These techniques are essential for determining the concentration of specific ions, the kinetics of redox reactions, and the stability of chemical compounds under varying conditions. By measuring changes in voltage, current, or resistance, it is possible to gain insights into chemical reactions and the nature of the substances involved.
One of the main advantages of these methods is their high sensitivity, which allows for the detection of minute quantities of substances. They also offer a non-destructive approach, making them suitable for analyzing delicate samples or materials that need to be preserved. Furthermore, these methods can be easily adapted to automated systems, making them ideal for routine analyses in various industries, including environmental monitoring, pharmaceutical testing, and food quality control.
Several common electrochemical techniques used for analysis include:
- Potentiometry: Measuring the potential difference between two electrodes to determine the concentration of ions in a solution.
- Coulometry: The measurement of the total charge passed during an electrochemical reaction, useful for determining the amount of a substance in a sample.
- Voltammetry: Analyzing the current response of a sample when a potential is applied, helping to identify the redox properties of various substances.
- Amperometry: Similar to voltammetry, but it focuses on measuring the current at a constant applied voltage, often used in biosensing applications.
These methods are not only effective for qualitative and quantitative analysis but are also pivotal in understanding complex chemical systems. By utilizing electrochemical techniques, researchers and professionals can gain precise data on molecular interactions, reaction mechanisms, and the electrochemical properties of materials.
Common Topics in Electrochemical Assessments
When preparing for assessments on topics related to the flow of electrons and the chemical processes that involve electrical energy, it’s important to focus on key concepts and practical applications. The goal is to not only understand theoretical principles but also to apply them to solve problems and explain reactions. Questions often test your ability to interpret electrochemical data, analyze reactions, and solve complex problems related to various types of cells and reactions.
Some of the most commonly explored topics include:
- Understanding Redox Reactions: You may be asked to identify oxidation and reduction half-reactions and calculate the overall cell potential for a given reaction.
- Cell Potential Calculations: Problems could involve calculating the voltage of a galvanic or electrolytic cell, given the standard electrode potentials of the involved species.
- Faraday’s Laws of Electrolysis: Questions often focus on calculating the amount of substance deposited or liberated at an electrode based on current and time.
- Standard Electrode Potentials: You might be required to predict the direction of current flow in a cell and determine the feasibility of a reaction.
- Applications of Electrochemical Principles: Understanding how principles apply in real-world scenarios, such as corrosion, energy storage, or biosensors.
These areas test both your theoretical knowledge and practical problem-solving abilities. Mastery of these concepts allows you to confidently tackle a wide range of challenges related to energy conversion, chemical analysis, and electrochemical reactions. Whether you’re required to balance equations, predict reaction outcomes, or calculate cell potentials, focusing on these fundamental concepts is essential for success.
Memorizing Key Electrochemical Equations
Mastering the essential equations involved in the study of reactions that convert chemical energy into electrical energy, and vice versa, is fundamental to understanding the underlying principles of this field. The ability to recall and apply these key formulas with ease can greatly enhance problem-solving efficiency and improve overall comprehension. These equations often serve as the foundation for calculating various parameters, such as cell potentials, reaction spontaneity, and the amount of substance involved in electrochemical processes.
To effectively memorize these crucial equations, it’s helpful to break them down into their core components and understand their significance. Some of the most important formulas include:
- Nernst Equation: Used to calculate the equilibrium potential for a redox reaction under non-standard conditions.
- Faraday’s Law of Electrolysis: Describes the relationship between the amount of substance altered at an electrode and the electric charge passed through the electrolyte.
- Cell Potential Formula: Helps calculate the voltage of a galvanic or electrolytic cell based on the standard electrode potentials of the half-reactions.
- Gibbs Free Energy Equation: Connects the free energy change of a reaction to the cell potential and can help predict whether a reaction is spontaneous.
To aid in memorization, consider using mnemonic devices or visual aids, such as diagrams, to reinforce the concepts. Regular practice through solving problems that require the use of these formulas will also improve retention and application skills. Additionally, focusing on understanding the logic behind each equation, rather than rote memorization alone, will provide a deeper comprehension of their relevance and usage in real-world scenarios.
Frequently Asked Questions in Electrochemistry
As you delve into the study of chemical reactions that generate electrical energy or utilize it, it’s common to encounter several recurring topics. These are questions that often arise due to the complex nature of the principles, calculations, and applications involved. Understanding the answers to these frequently discussed points can significantly improve clarity and problem-solving capabilities in this field. Below are some of the most commonly raised queries and their explanations.
What is the Nernst Equation used for?
The Nernst Equation is essential for calculating the potential of an electrochemical cell under non-standard conditions. It allows one to determine how the concentration of reactants and products affects the voltage, enabling predictions about reaction direction and feasibility in real-world scenarios. By incorporating temperature and ion concentration, it provides a more accurate reflection of conditions outside of standard states.
How do standard electrode potentials relate to reaction spontaneity?
Standard electrode potentials offer valuable insights into whether a redox reaction is spontaneous or not. By comparing the potentials of two half-reactions, we can determine the overall cell potential, which in turn indicates if the reaction will proceed in a given direction. A positive cell potential suggests that the reaction is spontaneous, while a negative value indicates the opposite.
These types of inquiries not only aid in better understanding theoretical concepts but also sharpen the ability to apply these principles effectively during practical problem-solving sessions. Familiarizing yourself with the most common concerns and solutions will help build a solid foundation in this area of study.
Tips for Electrochemistry Exam Preparation
Successfully preparing for assessments involving chemical reactions that generate or use electrical energy requires a focused and structured approach. To effectively tackle problems in this field, it’s essential to combine theoretical knowledge with practical application. Here are some strategies to help you organize your study sessions and maximize your performance.
Understand Core Concepts
Familiarizing yourself with the foundational principles is crucial for solving complex problems. Focus on the following areas:
- Redox reactions and their components
- Standard electrode potentials and their significance
- Understanding cell potentials and the Nernst equation
- The relationship between concentration and voltage in reactions
- Types of electrochemical cells and their applications
Practice Problem Solving
Repetition is key when mastering any technical subject. Here’s how to approach practice effectively:
- Solve problems from textbooks and online resources.
- Attempt questions with varying levels of difficulty to test your understanding.
- Time yourself to simulate real test conditions.
- Review your solutions to identify patterns and common mistakes.
By focusing on these techniques, you’ll enhance your ability to apply concepts quickly and accurately during assessments, ensuring you’re well-prepared when the time comes.
Resources for Revision

Effectively preparing for a deeper understanding of reactions involving electron movement and chemical processes requires the use of diverse resources. These materials help in mastering core principles, refining practical skills, and reinforcing theoretical knowledge. By utilizing various tools, learners can enhance their ability to grasp the intricacies of such topics and apply them effectively.
Essential Textbooks
Books remain a key resource for learning, providing comprehensive explanations, examples, and exercises. The following titles are recommended for anyone looking to strengthen their understanding of key concepts related to reaction mechanisms, energy transformations, and their applications:
| Book Title | Author | Focus Area |
|---|---|---|
| Physical Chemistry: A Molecular Approach | Donald A. McQuarrie | Thermodynamics, kinetics, and fundamental chemical processes |
| Principles of Modern Chemistry | David W. Oxtoby | Reaction dynamics, redox systems, and molecular interactions |
| Introduction to Chemical Engineering Kinetics | J.M. Smith | Kinetics, system optimization, and chemical engineering principles |
Online Platforms for Interactive Learning
In addition to traditional textbooks, digital platforms provide engaging and interactive ways to study. These resources allow learners to access videos, quizzes, and other materials designed to support self-paced learning:
- Khan Academy: Offers a wide range of video lessons and exercises, perfect for reinforcing fundamental concepts.
- Coursera: Provides online courses from top universities, featuring lectures, assignments, and peer feedback.
- Quizlet: A great tool for practicing and testing knowledge with flashcards covering key terms and concepts.
- ChemCollective: Offers virtual laboratories for conducting experiments and simulating real-world scenarios.
By integrating these resources into your study routine, you can build a strong foundation in the principles of chemical processes, develop problem-solving skills, and better prepare for real-world applications in this field.